What is in this leaflet
This leaflet answers some common questions about CHOLSTAT.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have benefits and risks. Your doctor has weighed the risks of you taking CHOLSTAT against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, talk to your doctor or pharmacist.
Keep this leaflet with your medicine. You may need to read it again.
What CHOLSTAT is used for
CHOLSTAT is used to:
- lower high blood cholesterol levels (doctors call this hypercholesterolaemia).
- treat people who have had a heart attack or an episode of unstable angina pectoris (chest pain), even when their cholesterol levels are normal.
- lower cholesterol in heart or kidney transplant patients, who are also being given immunosuppressive medicine.
- reduce the risk of further heart disease
- reduce the risk of having a stroke
- treat heterozygous familial hypercholesterolaemia in children and adolescent patients aged 8 years and older as an added measure to diet and lifestyle changes.
There are different types of cholesterol, called LDL and HDL. LDL cholesterol is the 'bad' cholesterol that can block your blood vessels. HDL cholesterol is the 'good' cholesterol that is thought to remove the 'bad' cholesterol from the blood vessels.
Cholesterol is present in many foods and is also made in your body by the liver. If your body does not balance the amount of cholesterol it needs with the amount of cholesterol eaten, then your cholesterol becomes too high.
High cholesterol is more likely to occur with certain diseases or if you have a family history of high cholesterol.
When you have high levels of cholesterol it may 'stick' to the inside of your blood vessels instead of being carried to the parts of the body where it is needed. Over time, this can form hard areas [called plaques] on the walls of your blood vessels, making it more difficult for the blood to flow. This blocking of your blood vessels can lead to heart disease (such as heart attack and angina), and stroke.
If you have had a heart attack, an episode of unstable angina or you have too much cholesterol in your blood, then you have an increased risk of a blood clot forming in your blood vessels and causing a blockage. Blood vessels that become blocked in this way can lead to further heart disease, angina or stroke.
CHOLSTAT tablets contain pravastatin sodium, a drug that reduces the level of cholesterol in your blood and helps to protect you in other ways from heart attack or stroke. It is more effective if it is taken with a diet low in fat.
Ask your doctor if you have any questions about why CHOLSTAT has been prescribed for you. Your doctor may have prescribed CHOLSTAT for another reason.
CHOLSTAT is available only with a doctor's prescription.
There is no evidence that CHOLSTAT is addictive.
Before you take CHOLSTAT
When you must not take it
Do not take CHOLSTAT if you are allergic to medicines containing pravastatin sodium, any other medicine used to reduce the level of cholesterol in your blood or any of the ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include skin rash, itching or hives; swelling of the face, lips or tongue which may cause difficulty in swallowing or breathing, wheezing or shortness of breath.
Do not take CHOLSTAT if you:
- have ever had liver problems
- have had muscle pains caused by any other medicine used to treat high cholesterol or triglycerides (fats).
Do not take CHOLSTAT if you are pregnant or planning to become pregnant. CHOLSTAT can harm your developing baby if you take it during pregnancy. Women who are able to conceive, should not take CHOLSTAT unless using effective contraception (e.g., the birth control pill).
Do not take CHOLSTAT if you are breastfeeding. CHOLSTAT passes into breast milk and can harm your baby.
Do not take CHOLSTAT if the expiry date (EXP.) printed on the pack has passed. If you take the tablets after the expiry date, they may not work as well.
Do not take CHOLSTAT if the packaging shows signs of tampering or the tablets do not look quite right.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you are allergic to any other medicines, foods, dyes or preservatives.
Let your doctor know if you are drinking alcohol while taking CHOLSTAT.
Tell your doctor if you have, or have had, any medical conditions, especially the following:
- liver problems
- kidney problems
- diabetes
- under-active thyroid
- suffer from central nervous system vascular lesions
- hormonal disorders
- increased triglycerides in blood
- homozygous familial hypercholesterolaemia
- increased triglycerides in your blood
- suffer from muscle disease (including pain, tenderness or weakness)
- are or may become pregnant
- are breastfeeding
Your doctor may want to take special care if you have or had any of these conditions.
Tell your doctor if you drink alcohol regularly.
Tell your doctor if you plan to have surgery.
If you have not told your doctor about any of the above, tell them before you start taking CHOLSTAT.
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you buy without a prescription from a pharmacy, supermarket or health food shop.
Some medicines may be affected by CHOLSTAT, or may affect how well it works. These include:
- any other medicine to lower cholesterol
- gemfibrozil and fenofibrate
- colestyramine and colestipol
- ciclosporin
- colchicine
- ketoconazole
- spironolactone
- cimetidine
- nicotinic acid
- erythromycin or clarithromycin
- antacids
- fusidic acid
- propranolol
- rifampicin
- digoxin
- warfarin or another coumarin anticoagulant.
Your doctor can tell you what to do if you are taking any of these medicines.
If you are not sure whether you are taking any of these medicines, check with your doctor or pharmacist.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking CHOLSTAT.
How to take CHOLSTAT
Follow all directions given to you by your doctor and pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the pack, ask your doctor or pharmacist.
How to take it
Swallow the tablets with a glass of water.
CHOLSTAT tablets should not be divided.
How much to take
The usual starting dose for lowering cholesterol is 10 mg to 20 mg at night, and varied ultimately between 10 mg to 80 mg once a day.
The dose for reducing the possibility of a stroke or heart attack is 40 mg per day.
The recommended dose is 20 mg once daily for children 8-13 years of age and 40 mg once daily in adolescents 14-18 years of age, with heterozygous familial hypercholesterolaemia.
However, the dose varies from patient to patient. Also, people over 65 may require a lower than usual dose. Therefore, your doctor will decide on the right dose for you after taking into consideration a number of factors including your cholesterol level and any other medicines that you are taking.
When to take it
Take CHOLSTAT once a day in the evening before bed-time. For the best result, take CHOLSTAT on an empty stomach (e.g., 3 hours after your dinner).
Take your medicine at about the same time each day. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
How long to take it for
Keep taking CHOLSTAT for as long as your doctor recommends. To properly control your condition, take CHOLSTAT every day. Treatment with CHOLSTAT is usually long term, even lifelong.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take the missed dose as soon as you remember, and then go back to taking your tablets as you would normally.
Do not take a double dose to make up for the dose you missed.
If you are not sure what to do or have any questions, ask your doctor or pharmacist.
If you take too much (overdose)
Immediately telephone your doctor, or the Poisons Information Centre (telephone 13 11 26), or go to Accident and Emergency at the nearest hospital, if you think you or anyone else may have taken too much CHOLSTAT. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
While you are taking CHOLSTAT
Things you must do
Before starting any new medicine, tell your doctor or pharmacist that you are taking CHOLSTAT.
Tell all the doctors, dentists and pharmacists who are treating you that you are taking CHOLSTAT.
Use effective contraception (e.g., birth control pill) while you are taking CHOLSTAT.
If you become pregnant while taking CHOLSTAT, tell your doctor immediately.
If you plan to have surgery, including dental surgery, tell your doctor or dentist that you are taking CHOLSTAT.
If you notice that you are getting muscle pain, tenderness or weakness for no reason, tell your doctor as soon as you can.
If you have to have any blood tests tell your doctor that you are taking CHOLSTAT. CHOLSTAT may affect the results of some tests.
Visit your doctor regularly so they can check on your progress. Your doctor may want to do some blood tests to check your liver.
Things you must not do
Do not stop taking CHOLSTAT, without checking with your doctor.
Do not use CHOLSTAT to treat any other conditions unless your doctor tells you to.
Do not give CHOLSTAT to anyone else, even if they have the same condition as you.
Things to be careful of
Be careful driving or operating machinery until you know how CHOLSTAT affects you. CHOLSTAT generally does not interfere with your ability to drive or operate machinery. However some people do experience dizziness. If this does occur to you, do not drive, operate machinery or do anything else that could be dangerous.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking CHOLSTAT.
Like all other medicines, CHOLSTAT may have unwanted side effects in some people. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor immediately or go to Accident and Emergency at the nearest hospital if you notice any of the following:
- skin rash, itching or hives
- swelling of the face, lips or tongue which may cause difficulty in swallowing or breathing, wheezing or shortness of breath.
The above list includes very serious side effects. You may need urgent medical attention or hospitalisation. These side effects are very rare.
You must tell your doctor immediately, or go to the hospital, if suffer any of the following:
- unexplained muscle pain, cramping tenderness and/or weakness (including eye and facial muscle)
- joint pain.
The above list includes serious side effects which may require medical attention. Serious side effects are rare.
It is also possible to suffer from an allergic reaction to CHOLSTAT, so tell your doctor if you develop a skin rash or itchiness, fever, joint pain or shortness of breath.
Tell your doctor if you notice any of the following and they worry you:
- nausea, vomiting
- upset stomach, wind, constipation, diarrhoea.
- headache, dizziness
- vision disturbance, ringing in the ears
- nervousness, sleep disturbance
- tiredness
- passing urine too often.
The above list includes the more common side effects of your medicine. They are usually mild and short-lived.
Tell your doctor if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some patients. Some changes can only be found when your doctor does tests from time to time to check your progress.
After taking CHOLSTAT
Storage
Keep CHOLSTAT where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Keep your tablets in a cool dry place where the temperature stays below 25°C.
Do not store CHOLSTAT or any other medicine in the bathroom or near a sink.
Do not leave CHOLSTAT in the car or on window sills. Heat and dampness can destroy some medicines.
Disposal
If your doctor tells you to stop taking CHOLSTAT, or your tablets have passed their expiry date, ask your pharmacist what to do with any that are left over.
Product description
What it looks like
CHOLSTAT comes in 3 strengths of tablets:
- CHOLSTAT 10 - white to off-white, capsule shaped, biconvex tablets with "G | G" on one side and "PR |10" on the other side
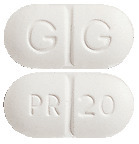
- CHOLSTAT 20 - white to off-white, capsule shaped, biconvex tablets with "G | G" on one side and "PR 20" on the other side
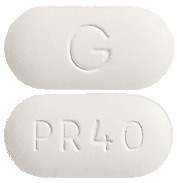
- CHOLSTAT 40 - white to off-white, capsule shaped, biconvex tablets with "G" on one side and "PR 40" on the other side.
Each pack contains 30 tablets.
Ingredients
The active ingredient in CHOLSTAT is pravastatin sodium.
Each CHOLSTAT 10 tablet contains 10 mg of pravastatin sodium.
Each CHOLSTAT 20 tablet contains 20 mg of pravastatin sodium.
Each CHOLSTAT 40 tablet contains 40 mg of pravastatin sodium.
The tablets also contain:
- aluminium magnesium silicate
- croscarmellose sodium
- povidone
- microcrystalline cellulose
- purified talc
- lactose monohydrate
- magnesium stearate.
CHOLSTAT contains soya bean products and sugars as lactose.
Manufacturer/Supplier
CHOLSTAT is supplied by:
Alphapharm Pty Ltd trading as Viatris
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.viatris.com.au
Phone: 1800 274 276
This leaflet was prepared in
November 2023.
Australian registration numbers:
CHOLSTAT 10 - AUST R 98486
CHOLSTAT 20 - AUST R 98488
CHOLSTAT 40 - AUST R 98489
CHOLSTAT® is a Viatris company trade mark
CHOLSTAT_cmi\Nov23/00
Published by MIMS December 2023



 There was no statistically significant difference in the change in lens opacity between the control and pravastatin treatment groups during this time interval.
There was no statistically significant difference in the change in lens opacity between the control and pravastatin treatment groups during this time interval. In a pooled analysis of two multicentre, double blind, placebo controlled studies in patients with primary hypercholesterolaemia, treatment with pravastatin at a daily dose of 80 mg increased HDL-C and significantly decreased total-C, LDL-C and TG from baseline after 6 weeks. The efficacy results of the individual studies were consistent with the pooled data. Mean percent changes from baseline after 6 weeks of treatment were: total-C (-27%), LDL-C (-37%), HDL-C (+3%) and TG (-19%), with placebo subtracted changes for LDL-C and TG of -36% and -20%, respectively.
In a pooled analysis of two multicentre, double blind, placebo controlled studies in patients with primary hypercholesterolaemia, treatment with pravastatin at a daily dose of 80 mg increased HDL-C and significantly decreased total-C, LDL-C and TG from baseline after 6 weeks. The efficacy results of the individual studies were consistent with the pooled data. Mean percent changes from baseline after 6 weeks of treatment were: total-C (-27%), LDL-C (-37%), HDL-C (+3%) and TG (-19%), with placebo subtracted changes for LDL-C and TG of -36% and -20%, respectively. The effect on the combined endpoint of coronary heart disease death or nonfatal myocardial infarction was evident as early as six months after beginning pravastatin therapy.
The effect on the combined endpoint of coronary heart disease death or nonfatal myocardial infarction was evident as early as six months after beginning pravastatin therapy.

 In the Long-Term Intervention with Pravastatin in Ischemic Disease (LIPID) study, the effect of pravastatin 40 mg daily was assessed in 9,014 men and women with normal to elevated serum cholesterol levels (baseline total-C = 4.0 to 7.0 mmol/L; mean total-C = 5.66 mmol/L; mean total-C/HDL-C ratio = 5.9) and who had experienced either a myocardial infarction or had been hospitalised for unstable angina pectoris in the preceding 3-36 months. Patients with a wide range of baseline levels of triglycerides were included (≤ 5.0 mmol/L) and baseline levels of HDL cholesterol did not restrict enrolment. At baseline, 82% of patients were receiving aspirin, 76% were receiving antihypertensive medication and 41% had undergone myocardial revascularisation. Patients in this multicentre, double blind, placebo controlled study participated for a mean of 5.6 years (median = 5.9 years). Treatment with pravastatin significantly reduced the risk for CHD death by 24% (p = 0.0004). The risk for coronary events (either CHD death or nonfatal MI) was significantly reduced by 24% (p < 0.0001) in the pravastatin treated patients. The risk for fatal or nonfatal myocardial infarction was reduced by 29% (p < 0.0001). Pravastatin reduced both the risk for total mortality by 23% (p < 0.0001) and cardiovascular mortality by 25% (p < 0.0001). The risk for undergoing myocardial revascularisation procedures (coronary artery bypass grafting or percutaneous transluminal coronary angioplasty) was significantly reduced by 20% (p < 0.0001) in the pravastatin treated patients. Pravastatin also significantly reduced the risk for stroke by 19% (p = 0.0477). Treatment with pravastatin significantly reduced the number of days of hospitalisation per 100 person years of follow-up by 15% (p < 0.001). The prespecified subgroup (age, sex, hypertensives, diabetics, smokers, lipid subgroups) analyses were conducted using the combined end point of CHD and nonfatal MI. The study was not powered to examine results within each subgroup but formal testing for heterogeneity of treatment effect was undertaken across each of the subgroups and no significant heterogeneity was found (p ≥ 0.08) i.e. a consistent treatment effect was seen with pravastatin therapy across all patient subgroups and event parameters. Among patients who qualified with a history of myocardial infarction, pravastatin significantly reduced the risk for total mortality by 25% (p = 0.0016); for CHD mortality by 23% (p = 0.004); for CHD events by 22% (p = 0.002) and for fatal or nonfatal MI by 25% (p = 0.0008). Among patients who qualified with a history of hospitalisation for unstable angina pectoris, pravastatin significantly reduced the risk for total mortality by 26% (p = 0.0035; for CHD mortality by 26% (p = 0.0358); for CHD events by 29% (p = 0.0001) and for fatal or nonfatal MI by 37% (p = 0.0003). (See Table 7.)
In the Long-Term Intervention with Pravastatin in Ischemic Disease (LIPID) study, the effect of pravastatin 40 mg daily was assessed in 9,014 men and women with normal to elevated serum cholesterol levels (baseline total-C = 4.0 to 7.0 mmol/L; mean total-C = 5.66 mmol/L; mean total-C/HDL-C ratio = 5.9) and who had experienced either a myocardial infarction or had been hospitalised for unstable angina pectoris in the preceding 3-36 months. Patients with a wide range of baseline levels of triglycerides were included (≤ 5.0 mmol/L) and baseline levels of HDL cholesterol did not restrict enrolment. At baseline, 82% of patients were receiving aspirin, 76% were receiving antihypertensive medication and 41% had undergone myocardial revascularisation. Patients in this multicentre, double blind, placebo controlled study participated for a mean of 5.6 years (median = 5.9 years). Treatment with pravastatin significantly reduced the risk for CHD death by 24% (p = 0.0004). The risk for coronary events (either CHD death or nonfatal MI) was significantly reduced by 24% (p < 0.0001) in the pravastatin treated patients. The risk for fatal or nonfatal myocardial infarction was reduced by 29% (p < 0.0001). Pravastatin reduced both the risk for total mortality by 23% (p < 0.0001) and cardiovascular mortality by 25% (p < 0.0001). The risk for undergoing myocardial revascularisation procedures (coronary artery bypass grafting or percutaneous transluminal coronary angioplasty) was significantly reduced by 20% (p < 0.0001) in the pravastatin treated patients. Pravastatin also significantly reduced the risk for stroke by 19% (p = 0.0477). Treatment with pravastatin significantly reduced the number of days of hospitalisation per 100 person years of follow-up by 15% (p < 0.001). The prespecified subgroup (age, sex, hypertensives, diabetics, smokers, lipid subgroups) analyses were conducted using the combined end point of CHD and nonfatal MI. The study was not powered to examine results within each subgroup but formal testing for heterogeneity of treatment effect was undertaken across each of the subgroups and no significant heterogeneity was found (p ≥ 0.08) i.e. a consistent treatment effect was seen with pravastatin therapy across all patient subgroups and event parameters. Among patients who qualified with a history of myocardial infarction, pravastatin significantly reduced the risk for total mortality by 25% (p = 0.0016); for CHD mortality by 23% (p = 0.004); for CHD events by 22% (p = 0.002) and for fatal or nonfatal MI by 25% (p = 0.0008). Among patients who qualified with a history of hospitalisation for unstable angina pectoris, pravastatin significantly reduced the risk for total mortality by 26% (p = 0.0035; for CHD mortality by 26% (p = 0.0358); for CHD events by 29% (p = 0.0001) and for fatal or nonfatal MI by 37% (p = 0.0003). (See Table 7.)
 The safety and efficacy of pravastatin doses above 40 mg daily have not been studied in children. The long-term efficacy of pravastatin therapy in childhood to reduce morbidity and mortality in adulthood has not been established.
The safety and efficacy of pravastatin doses above 40 mg daily have not been studied in children. The long-term efficacy of pravastatin therapy in childhood to reduce morbidity and mortality in adulthood has not been established. Molecular formula: C23H35NaO7.
Molecular formula: C23H35NaO7.