What is in this leaflet
This leaflet answers some common questions about this medicine.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking this medicine against the benefits he/she expects it will have for you.
If you have any concerns about taking this medicine, talk to your doctor or pharmacist.
Read this leaflet carefully before you start CINACALCET VIATRIS and keep it with the medicine.
You may need to read it again.
What CINACALCET VIATRIS is used for
CINACALCET VIATRIS is used to treat:
- a condition called secondary hyperparathyroidism (high-perpara-THIGH-royd-izm) in people with kidney disease who require dialysis treatment.
- a condition called primary hyperparathyroidism when surgical removal of the parathyroid gland is not a treatment option.
- high blood calcium levels in people with cancer of the parathyroid gland.
Secondary hyperparathyroidism
Kidney disease can cause a condition called secondary hyperparathyroidism, which can have a big impact on your health. Four small glands located behind the thyroid gland in your neck are called parathyroid glands. They make a hormone called parathyroid hormone (PTH). Normally, PTH makes sure you have just enough calcium and phosphorus in your blood to keep your bones, heart, muscles, nerves and blood vessels working well. When your kidneys are working properly, PTH keeps your calcium and phosphorus levels normal by moving the right amounts of calcium and phosphorus in and out of your bones.
When your kidneys aren't working properly, the calcium and phosphorus balance in your body is upset, and your parathyroid glands send out too much PTH to your body. This condition is called secondary hyperparathyroidism, and it can cause bone disease and also may be a risk factor for heart disease and abnormal calcium deposits in blood vessels and other parts of the body. This medicine lowers PTH by telling your parathyroid glands to stop releasing too much PTH into your blood. It also lowers your calcium and phosphorus levels.
Primary hyperparathyroidism/ Cancer of the parathyroid gland
An overactive parathyroid gland results in a condition called primary hyperparathyroidism, which can impact your health. Four small glands located behind the thyroid gland in your neck are called parathyroid glands. They make a hormone called parathyroid hormone (PTH). When your parathyroid glands are working normally, PTH keeps your calcium levels normal by moving the right amounts of calcium in and out of your bones.
Primary hyperparathyroidism is caused by an enlargement of one or more of the parathyroid glands occasionally due to cancer of the parathyroid gland. In primary hyperparathyroidism, your parathyroid glands send out too much PTH to your body and your blood level of calcium becomes high. This medicine lowers PTH by telling your parathyroid glands to stop releasing too much PTH into your blood. It also lowers your calcium and phosphorus levels.
Your doctor may have prescribed this medicine for another reason. Ask your doctor if you have any questions about why this medicine has been prescribed for you.
Before you take CINACALCET VIATRIS
When you must not take it
Do not take CINACALCET VIATRIS if you have an allergy to:
- any medicine containing cinacalcet
- any of the ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin
Do not give this medicine to a child.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you have or have had any of the following medical conditions:
- seizures (sometimes called fits or convulsions, see 'Side effects' section)
- heart failure (or a heart condition, see 'Side effects' section)
- intolerance to sugars (sometimes called lactose intolerance, see 'Ingredients' section)
- ulcers (stomach or intestinal), serious vomiting, inflammation of the stomach and swallowing tube
Tell your doctor if you are pregnant or plan to become pregnant or are breast-feeding. Your doctor can discuss with you the risks and benefits involved.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Tell your doctor if you are taking ketoconazole, erythromycin, itraconazole, rifampicin, phenytoin, amitriptyline, flecainide, vinblastine, thioridazine or medicines known as tricyclic antidepressants. Some of these medicines can affect how this medicine works, while others are affected by this medicine.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
How to take CINACALCET VIATRIS
This medicine must be taken orally either with, or shortly after food. The tablets must be taken whole and are not to be divided. Follow all directions given to you by your doctor, nurse and pharmacist carefully. They may differ from the information in this leaflet.
If you do not understand the instructions below, ask your doctor, nurse or pharmacist for help.
How much to take
If you are being treated for secondary hyperparathyroidism
The usual starting dose for this medicine is 30 mg (one tablet) once per day.
Your doctor will take regular blood samples to measure how you are responding to this medicine and will adjust your dose as necessary in order to control your parathyroid hormone, calcium and phosphate levels.
Once your condition is under control, your doctor will continue to regularly check your blood and your dose may be adjusted further in order to maintain long-term control of your parathyroid hormone, calcium and phosphate levels.
If you are being treated for primary hyperparathyroidism/ cancer of the parathyroid gland
The usual starting dose for this medicine is 30 mg (one tablet) twice per day.
Your doctor will take regular blood samples to measure how you are responding to this medicine and will adjust your dose as necessary in order to control your calcium levels.
Once your condition is under control, your doctor will continue to regularly check your blood and your dose may be adjusted further in order to maintain long-term control of your calcium levels.
If you forget to take this medicine
Skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember, and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for the dose that you missed.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much this medicine. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
If you do not understand the instructions on the box, ask your doctor or pharmacist for help.
While you are using CINACALCET VIATRIS
Things you must do
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking this medicine.
Tell any other doctors, dentists and pharmacists who treat you that you are taking this medicine.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking this medicine. It may affect other medicines used during surgery.
If you become pregnant while taking this medicine, tell your doctor immediately.
If you are about to have any blood tests, tell your doctor that you are taking this medicine. It may interfere with the results of some tests.
Keep all of your doctor's appointments so that your progress can be checked.
Things you must not do
Do not take CINACALCET VIATRIS to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Things to be careful of
Be careful driving or operating machinery until you know how CINACALCET VIATRIS affects you. This medicine may cause dizziness, and seizures in some people. If you have any of these symptoms, do not drive, operate machinery or do anything else that could be dangerous.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking CINACALCET VIATRIS.
This medicine helps most people, but it may have unwanted side effects in a few people. All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
CINACALCET VIATRIS lowers your calcium level. If calcium levels become too low, you might get hypocalcaemia. Signs of hypocalcaemia include numbness or tingling around the mouth, muscle aches or cramps and seizures. If you have any of these symptoms, you should tell your doctor straight away. People with kidney disease not requiring dialysis are at increased risk of developing hypocalcaemia.
If any of the following happens, stop taking this medicine and go straight to the hospital, as you may need urgent medical attention:
- Allergic reaction
- Skin rash over a large area of the body
- Swelling of the face, lips, mouth, tongue or throat which may cause difficulty in swallowing or breathing
- Shortness of breath
- Wheezing
- Faintness, rapid pulse or sweating
Tell your doctor, nurse or pharmacist if you notice any of the following and they worry you:
- Nausea and vomiting
People taking this medicine may have a greater chance of developing nausea and/or vomiting. If nausea or vomiting is making it difficult to take your medicines or is of any concern to you, you should tell your doctor. - Seizure (also known as a fit or convulsion)
The risk of having seizures is greater in people who have had seizures before. Lowering the calcium level too much may also increase the risk of having a seizure. If you have a seizure you should tell your doctor straight away. - Dizziness or light headedness, or worsening of a heart condition
This medicine may cause low blood pressure or affect the heart's function in people who have a heart condition (heart failure). If you know you have a heart condition, tell your doctor. - Rash
Pinkish, itchy swellings on the skin, also called hives or nettle rash. Itchy rash. - Bleeding from the stomach or intestines
Bleeding from the stomach or intestines may occur in some patients. You may experience severe pain or tenderness in the stomach, vomit blood or material that looks like coffee grounds, or have black or tar-like stools.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Other side effects not listed above may occur in some people.
After using this CINACALCET VIATRIS
Storage
Keep your tablets in a cool dry place where the temperature stays below 25°C.
Do not store CINACALCET VIATRIS or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
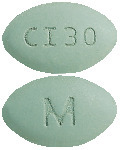
CINACALCET VIATRIS 30 mg tablets:
green, film-coated, oval, biconvex, bevelled edge tablet debossed with M on one side of the tablet and CI30 on the other side.
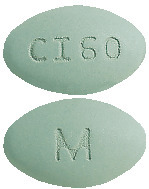
CINACALCET VIATRIS 60 mg tablets:
green, film-coated, oval, biconvex, bevelled edge tablet debossed with M on one side of the tablet and CI60 on the other side.
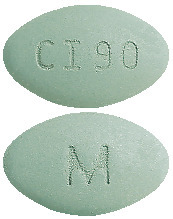
CINACALCET VIATRIS 90 mg tablets:
green, film-coated, oval, biconvex, bevelled edge tablet debossed with M on one side of the tablet and CI90 on the other side.
Ingredients
CINACALCET VIATRIS contains either 30 mg, 60 mg, or 90 mg of cinacalcet as the active ingredient.
The tablets also contain the following inactive ingredients:
- microcrystalline cellulose
- colloidal anhydrous silica
- crospovidone
- povidone
- magnesium stearate
- OPADRY complete film coating system 03K51674 GREEN (ARTG PI No: 109807).
CINACALCET VIATRIS contains sulfites.
Distributor
CINACALCET VIATRIS is distributed in Australia by:
Alphapharm Pty Ltd trading as Viatris
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.viatris.com.au
Phone: 1800 274 276
This leaflet was prepared in December 2022.
Australian registration numbers:
AUST R 289379 (30 mg)
AUST R 289380 (60 mg)
AUST R 289381 (90 mg)
CINACALCET VIATRIS_cmi\Dec22/00
Published by MIMS February 2023

 The incidence of serious adverse events (29% vs 31%) and deaths (2% vs 3%) was similar in the cinacalcet and placebo groups, respectively.
The incidence of serious adverse events (29% vs 31%) and deaths (2% vs 3%) was similar in the cinacalcet and placebo groups, respectively. Mean iPTH and Ca x P by treatment group for the overall study population during the 6-month treatment period are presented in Figure 1 and Figure 2.
Mean iPTH and Ca x P by treatment group for the overall study population during the 6-month treatment period are presented in Figure 1 and Figure 2.
 In patients receiving cinacalcet, reductions in iPTH and Ca x P occurred within 2 weeks and were maintained for at least 12 months of treatment (n = 99 on cinacalcet, 111 on placebo).
In patients receiving cinacalcet, reductions in iPTH and Ca x P occurred within 2 weeks and were maintained for at least 12 months of treatment (n = 99 on cinacalcet, 111 on placebo).
 Whilst a 15 mg dose of cinacalcet was used in the paediatric PK study, this dose strength is not registered.
Whilst a 15 mg dose of cinacalcet was used in the paediatric PK study, this dose strength is not registered. Chemical name: N-[1-(R)-(1-naphthyl) ethyl]-3-[3-(trifluoromethyl) phenyl]-1-aminopropane hydrochloride.
Chemical name: N-[1-(R)-(1-naphthyl) ethyl]-3-[3-(trifluoromethyl) phenyl]-1-aminopropane hydrochloride.