What is in this leaflet
This leaflet answers some common questions about Ciprofloxacin GH.
This leaflet does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking Ciprofloxacin GH against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What Ciprofloxacin GH is used for
Ciprofloxacin GH is used for the treatment of infections of the lungs, skin, bones, joints, kidneys, bladder, prostate and bowel. Ciprofloxacin GH is also used to treat inhalational anthrax (an infection caused by breathing in the spores of bacteria).
Ciprofloxacin GH contains the active ingredient ciprofloxacin (present as hydrochloride monohydrate) which is an antibiotic belonging to a group of medicines called quinolones (pronounced kwin-o-lones). These antibiotics work by killing the bacteria that are causing your infection.
Ciprofloxacin GH will not work against infections caused by viruses such as colds or the flu.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
Before you take Ciprofloxacin GH
When you must not take it
Do not take Ciprofloxacin GH if you have an allergy to:
- ciprofloxacin, the active ingredient in Ciprofloxacin GH;
- any of the ingredients listed at the end of this leaflet;
- other medicines belonging to the quinolone chemical family (eg. moxifloxacin, norfloxacin, nalidixic acid).
Some of the symptoms of an allergic reaction may include:
- shortness of breath;
- wheezing or difficulty breathing;
- swelling of the face, lips, tongue or other parts of the body;
- rash, itching or hives on the skin.
Do not take Ciprofloxacin GH if you are also taking a medicine called tizanidine a muscle relaxant used to treat spasticity associated with multiple sclerosis, injury or diseases of the spinal cord. Ciprofloxacin GH can interfere with tizanidine and can lead to undesirable side effects.
Do not take this medicine after the expiry date printed on the pack and blister. The expiry date is printed on the carton and on each blister (eg. EXP 11-09 refers to November 2009). The expiry date refers to the last day of that month. If it has expired return it to your pharmacist for disposal.
Do not take this medicine if the packaging is torn or shows signs of tampering. If the packaging is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you are pregnant or plan to become pregnant. Ciprofloxacin GH is not recommended if you are pregnant, but your doctor will assess the benefit if required. Medicines similar to Ciprofloxacin GH have caused joint disease in immature animals.
Tell your doctor if you are breastfeeding. Ciprofloxacin GH is excreted into the breast milk. Your doctor will tell you whether you should take it and temporarily stop breastfeeding while you are taking the tablets.
Ciprofloxacin GH is not recommended in children under 18 years of age except for use in inhalation anthrax.
Ciprofloxacin GH should be used with caution in elderly patients as they are more prone to side effects.
Tell your doctor if you:
- suffer from epilepsy (seizures, convulsions), have had a stroke, or have kidney or liver disease.
- have arrhythmias (fast or irregular heartbeats). Ciprofloxacin GH may increase the risk of arrhythmias, especially in the elderly or patients with low potassium levels.
- have previously taken corticosteroids. You may be at increased risk of swelling of the tendons. Symptoms include pain, tenderness and sometimes restricted movement.
- have myasthenia gravis, a condition where the muscles become weak. Ciprofloxacin GH can worsen the symptoms of this condition.
- have a history of tendon disorders with the use of quinolones (eg. moxifloxacin, norfloxacin, nalidixic acid);
- have or have had a mental illness;
- have diabetes.
If you have not told your doctor or pharmacist about any of the above, tell them before you start taking Ciprofloxacin GH.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may be affected by Ciprofloxacin GH. These include:
- medicines used to treat arrhythmias (fast or irregular heartbeats);
- theophylline, a medicine used to treat asthma;
- oral anticoagulants, warfarin and its derivatives, medicines used to stop blood clots;
- phenytoin, a medicine used to treat epilepsy;
- oral antidiabetic agents;
- didanosine, a medicine used to treat viral infections;
- cyclosporin, a medicine used to suppress the immune system;
- non-steroidal anti-inflammatory drugs (NSAIDs), medicines used to treat pain, arthritis and other inflammatory conditions;
- methotrexate, a medicine used to treat certain types of cancers, severe psoriasis or severe rheumatoid arthritis;
- duloxetine, a medicine used to treat depression, anxiety, and nerve pain in people with diabetes;
- clozapine, a medicine used to treat schizophrenia;
- ropinirole, a medicine used to treat Parkinson’s disease or restless legs syndrome;
- the local anaesthetic lidocaine, a medicine used to numb pain or cause loss of sensation;
- oxpentifylline, a medicine used to treat circulation disorders;
- sildenafil, a medicine used to treat erectile dysfunction;
- agomelatine, a medicine used to treat depression;
- zolpidem, a medicine used to treat sleep disorders.
These medicines may be affected by Ciprofloxacin GH or may affect how well it works. You may need different amounts of your medicine, or you may need to take different medicines.
Some medicines may interfere with the absorption of Ciprofloxacin GH. These include:
- multivitamins, mineral supplements, antacids (used for indigestion) and other medicines containing iron, zinc, magnesium, aluminium or calcium;
- sucralfate, a medicine used to treat duodenal or stomach ulcers;
- medicines used to treat HIV infection;
- probenecid, a medicine used to treat gout;
- omeprazole, a medicine used to treat stomach ulcers and other conditions where stomach produces too much acid;
- sevelamer, a medicine used to treat high blood levels of phosphorus in patients with kidney disease who are on dialysis;
- metoclopramide, a medicine used to relieve nausea and vomiting; heartburn, and stomach pain.
You can still take these medicines while you are taking Ciprofloxacin GH. However, you must take Ciprofloxacin GH at least 2 hours before or 2 hours after taking any of these medicines. Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
How to take Ciprofloxacin GH
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions printed on the pharmacist label, ask your doctor or pharmacist for help.
How much to take
Your doctor or pharmacist will tell you how much and how often you should take Ciprofloxacin GH. This will depend on the type of infection and any medical conditions you may have.
The usual adult dosage for most infections is one tablet twice daily for 7 to 14 days. You may need to take your tablets for a longer period for some types of infection. The dose will be determined by your doctor as it depends upon the type of infection you have.
When to take it
Ciprofloxacin GH tablets are usually taken twice a day. Take your tablets at the same time each day preferably on an empty stomach. However, they can be taken with or without food.
How long to take it
The length of treatment may vary from 1 to 28 days or longer depending on the type of infection.
Continue taking Ciprofloxacin GH until you have finished the blister pack or for as long as your doctor tells you. Do not stop taking your tablets because you are feeling better.
If you do not complete the full course prescribed by your doctor, the infection may not clear completely or your symptoms may return.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to. Otherwise, take it as soon as you remember, and then go back to taking it as you would normally.
Do not take a double dose to make up for the dose that you missed.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering when to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor, or the Poisons Information Centre (telephone in Australia 13 11 26, in New Zealand 0800 POISON or 0800 764 766), or go to the Accident and Emergency department at your nearest hospital, if you think you or anyone else may have taken too much Ciprofloxacin GH. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
While you are taking it
Things you must do
Tell all the doctors, dentists and pharmacists who are treating you that you are taking Ciprofloxacin GH.
Tell your doctor if you need to have a surgical or dental procedure that you are taking Ciprofloxacin GH.
Ciprofloxacin GH may affect the results of certain laboratory tests. If you are about to have any tests, tell your doctor that you are taking this medicine.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking Ciprofloxacin GH.
Drink plenty of water while you are taking Ciprofloxacin GH. This helps to stop crystals forming in your urine.
If you become pregnant while you are taking Ciprofloxacin GH, tell your doctor immediately.
If you develop diarrhoea, tell your doctor or pharmacist immediately. Do it even if it occurs several weeks after you have stopped taking Ciprofloxacin GH. Diarrhoea may mean that you have a serious condition affecting your bowel. You may need urgent medical care. Do not take any medications for diarrhoea without checking with your doctor or pharmacist.
Tell your doctor immediately if you experience symptoms of depression or self-endangering behaviour. Ciprofloxacin should be discontinued immediately.
Tell your doctor immediately if you develop pain, burning, tingling, numbness or weakness in any part of the body. Ciprofloxacin GH should be discontinued immediately.
Things you must not do
Do not give your Ciprofloxacin GH tablets to anyone else, even if they have the same condition as you.
Do not use Ciprofloxacin GH to treat other conditions unless your doctor tells you to.
Do not stop taking your tablets because you are feeling better, unless your doctor told you to do so.
If you do not complete the full course prescribed by your doctor, some of the bacteria causing your infection may not be killed. These bacteria may continue to grow and multiply so that your infection may not clear up completely or it may return.
Things to be careful of
Avoid excessive exposure to direct sunlight. Your skin may become more prone to sunburn. If such a reaction occurs, stop taking Ciprofloxacin GH immediately and tell your doctor.
Be careful driving or operating machinery until you know how Ciprofloxacin GH affects you. Ciprofloxacin GH tablets may cause dizziness in some patients, especially after the first few doses. Your ability to drive and/or operate machinery may be impaired. If you drink alcohol while taking this medicine, dizziness may be worse.
Ciprofloxacin GH tablets may increase the stimulatory effects of caffeine.
Side Effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Ciprofloxacin GH.
All medicines have side effects. Sometimes they are serious, most of the time they are not. In serious cases, you may need medical attention.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you notice any of the following and they worry you:
- nausea;
- diarrhoea.
These are the common side effects of Ciprofloxacin GH. They are usually mild and short-lived.
Tell your doctor immediately, or go to the Accident and Emergency department at your nearest hospital if you notice any of the following:
- severe skin rashes, peeling of the skin and/or mucosal reactions;
- signs of allergy such as rash, swelling of the face, lips, mouth, throat or other parts of the body, shortness of breath, wheezing or trouble breathing;
- fainting;
- yellowing of the skin and eyes, also called jaundice;
- severe watery or bloody diarrhoea, even if it occurs several weeks after taking your tablets;
- fits (seizures, convulsions);
- confusion, nightmares, hallucinations, and psychotic reaction (even progressing to self-endangering behaviour);
- fast or irregular heart beats;
- visual disturbances (eyesight problems);
- ringing in the ear, loss of hearing;
- abdominal pain/cramps. Very rarely this can progress to a serious condition accompanied by fever and fatigue;
- pain, burning, tingling, numbness and/or weakness in your limbs.
These serious side effects are rare. If you have them, you may need urgent medical attention.
In isolated instances, some serious side effects may be long-lasting (>30 days) and disabling, such as tendonitis, tendon rupture, musculoskeletal disorders and other reactions affecting the nervous system including mental health disorders and disturbance of senses.
Photosensitivity (getting sunburnt very easily) can occasionally occur with Ciprofloxacin GH. However, it is temporary and staying out of direct sunlight while on Ciprofloxacin GH will prevent it from happening.
Rarely, there can be a worsening of the symptoms of myasthenia gravis. This is a condition in which the muscles become weak and tire easily, causing drooping eyelids, double vision, difficulty in speaking and swallowing and sometimes muscle weakness in the arms or legs.
Rarely, the Achilles tendon (extending from the calf to the heel of the foot) or other tendons have been torn after Ciprofloxacin GH therapy. This may occur even within the first 48 hours of treatment and up to several months after completing treatment with Ciprofloxacin GH. This risk of tendon damage may be increased in elderly patients, during strenuous physical activity, if you are currently being treated with a type of medicine called corticosteroids, if you have reduced kidney function or have received solid organ transplants. Tell your doctor immediately if you feel any discomfort, pain or inflammation of a tendon.
Rarely, you may experience hyperglycaemia (high blood sugar), or hypoglycaemia (low blood sugar). Symptoms of hyperglycaemia include increased thirst, appetite and urination. Symptoms of hypoglycaemia include weakness, shaking, sweating, light headedness, headache, behavioural changes, confusion, numbness/pins and needles in the lips, fingers or toes, irritability and hunger.
Tell your doctor if you experience these symptoms.
If you experience any of these symptoms during treatment with Ciprofloxacin GH tablets, tell your doctor or pharmacist immediately. Ciprofloxacin GH may need to be discontinued.
Tell your doctor or pharmacist if you notice anything else that is making you feel unwell. Other side effects not listed above may also occur in some patients.
After taking Ciprofloxacin GH
Storage
Keep your tablets in the blister pack until it is time to take them. If you take the tablets out of the box or the blister pack they may not keep well.
Keep your tablets in a cool dry place where the temperature stays below 25°C.
Do not store Ciprofloxacin GH or any other medicine in the bathroom, near a sink or on a window sill.
Do not leave it in the car. Heat and damp can destroy some medicines.
Keep your tablets where children cannot reach them. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking Ciprofloxacin GH tablets or the tablets have passed their expiry date, ask your pharmacist what to do with any that are left over.
Return any unused medicine to your pharmacist.
Product description
What it looks like
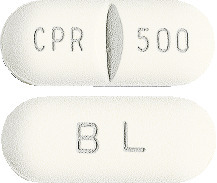
Ciprofloxacin GH 500 mg tablets: White to creamish white, capsule shaped film-coated tablets, embossed with “CPR 500” and a break line on one side and “BL” on the reverse.
Ciprofloxacin GH 500 mg tablets are supplied in blister packs of 14 tablets.
Ingredients
Active ingredient
Ciprofloxacin GH 500 mg tablets contain 500 mg ciprofloxacin (as hydrochloride monohydrate).
Other ingredients
- maize starch;
- microcrystalline cellulose;
- sodium starch glycollate;
- colloidal anhydrous silica;
- magnesium stearate;
- hypromellose;
- purified talc;
- titanium dioxide;
- macrogol 4000.
Australian Registration Numbers
Ciprofloxacin GH 500 mg tablets: AUST R 127781.
Sponsor
Generic Health Pty Ltd
Suite 2, Level 2
19-23 Prospect Street
Box Hill VIC 3128
E-mail: [email protected]
Telephone: +61 3 9809 7900
Website: www.generichealth.com.au
This leaflet was prepared in February 2023.
Published by MIMS April 2023


 ADRs derived from postmarketing reports (status: 31 July 2005) for which a frequency could not be estimated are listed in Table 2.
ADRs derived from postmarketing reports (status: 31 July 2005) for which a frequency could not be estimated are listed in Table 2. In isolated instances, some serious adverse drug reactions may be long-lasting (> 30 days) and disabling; such as tendonitis, tendon rupture, musculoskeletal disorders, and other reactions affecting the nervous system including psychiatric disorders and disturbance of senses.
In isolated instances, some serious adverse drug reactions may be long-lasting (> 30 days) and disabling; such as tendonitis, tendon rupture, musculoskeletal disorders, and other reactions affecting the nervous system including psychiatric disorders and disturbance of senses.
 Maximum serum concentrations are attained 1 to 2 hours after oral dosing. Mean concentrations 12 hours after dosing with 250, 500 or 750 mg are 0.1, 0.2 and 0.4 microgram/mL respectively.
Maximum serum concentrations are attained 1 to 2 hours after oral dosing. Mean concentrations 12 hours after dosing with 250, 500 or 750 mg are 0.1, 0.2 and 0.4 microgram/mL respectively. Chemical Name: (1-cyclopropyl-6-fluoro-1,4- dihydro-4-oxo-7- (1-piperazinyl)-3-quinolinecarboxylic acid).
Chemical Name: (1-cyclopropyl-6-fluoro-1,4- dihydro-4-oxo-7- (1-piperazinyl)-3-quinolinecarboxylic acid).