What is in this leaflet
This leaflet answers some common questions about CYTOTEC.
It does not contain all of the available information.
It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking CYTOTEC against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What CYTOTEC is used for
- To treat acute ulcers in the stomach (gastric ulcers), or in the first part of the small intestine (duodenal ulcers).
- To prevent development of stomach ulcers, which may sometimes be caused by arthritis medicines that are called non-steroidal anti-inflammatory drugs (NSAIDs).
- To prevent bleeding in the stomach or upper intestine in hospital patients after surgery.
CYTOTEC makes your stomach produce less acid, and it helps your stomach protect itself against damage from acid and certain other substances, such as NSAIDs.
The active ingredient in CYTOTEC is called misoprostol. Misoprostol is very similar to a group of substances called prostaglandins, which occur naturally in the stomach and other parts of the body. When the amount of these natural prostaglandins is lower than normal, there is a risk that ulcers may occur in the stomach or duodenum. This reduction in prostaglandins is often a side effect of NSAIDs. CYTOTEC can replace prostaglandins and help to prevent ulcers, or help heal the ulcer if you already have one. If you are taking an NSAID, CYTOTEC helps protect your stomach while you continue to receive the benefit of pain relief and reduction in joint swelling from your arthritis medicine.
Your doctor, however, may prescribe CYTOTEC for another purpose.
Ask your doctor if you have any questions about why CYTOTEC has been prescribed for you.
There is no evidence that CYTOTEC is addictive.
Before you take CYTOTEC
When you must not use it
Do not take CYTOTEC if:
- you are allergic to CYTOTEC (or another prostaglandin medicine) or any of the tablet ingredients listed at the end of this leaflet.
If you have an allergic reaction you may get a skin rash, difficulty in breathing, hayfever or faintness. - you are pregnant, or there is a possibility you may be pregnant, or if you intend to become pregnant.
Tell your doctor if you become pregnant while you are taking CYTOTEC. The effects of CYTOTEC may be harmful to a developing baby (fetus).
If it is possible for you to become pregnant, you should use adequate contraception while you are taking CYTOTEC. Examples of adequate contraception are oral contraceptives ("the pill") or intra-uterine devices (IUDs).
CYTOTEC must not be used by pregnant women as it may cause miscarriage, and this could lead to potentially dangerous bleeding, hospitalisation, surgery, infertility or death. You should not become pregnant while taking CYTOTEC.
Do not use CYTOTEC after the expiry date (EXP) printed on the pack. It may have no effect at all, or worse, an entirely unexpected effect if you use it after the expiry date.
Do not use CYTOTEC if the packaging shows signs of tampering.
Do not use it to treat any other complaints unless your doctor says to.
Do not give this medicine to anyone else, even if their symptoms seem similar to yours.
Before you start to use it:
You must tell your doctor if:
- You are allergic to any other medicines or any foods, dyes or preservatives
- you have any other medical conditions, especially:
- epilepsy
- asthma
- diseases of the heart or blood vessels.
- bowel disease
- you are taking any other medicines, including medicines that you buy without a prescription from a pharmacy, supermarket or health food shop.
- you are breast feeding.
CYTOTEC passes into breast milk, therefore it is not recommended that you take CYTOTEC if you are breast feeding.
Use in children.
The effects of CYTOTEC have only been studied in adults, and there is no specific information comparing its use in children with use in adults.
How to take CYTOTEC
The usual dosage of CYTOTEC is one tablet two, three or four times a day.
CYTOTEC is best taken with food. Do not take CYTOTEC on an empty stomach.
Follow your doctor's instructions exactly, on how much CYTOTEC to take, and for how long to take it. If you are taking an NSAID, CYTOTEC may be prescribed for as long as you are taking the NSAID, whether or not you have stomach pain or other symptoms of ulcers. Some ulcers are painless, particularly those caused by NSAIDs.
If you miss a dose
If you miss a dose of CYTOTEC, take it as soon as possible. However, if it is almost time for your next dose, skip the missed dose and go back to your regular dosing schedule.
Do not take two doses together.
If you have trouble remembering when to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose):
If you think that you or anyone else may have taken too much CYTOTEC, immediately telephone your doctor or Poisons Information Centre (telephone Australia 13 11 26 or New Zealand 0800 POISON or 0800 764 766) for advice, or go to Accident and Emergency at your nearest hospital. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
Keep telephone numbers for these places handy.
Signs that may indicate an overdose of CYTOTEC include: sedation (feeling sleepy), shaking, fits, shortness of breath, stomach pains, diarrhoea, contraction of the uterus (womb), heart palpitations, low blood pressure or slow heart beat.
While you are using CYTOTEC
Things you must do
Use CYTOTEC exactly as your doctor has prescribed.
Stop taking CYTOTEC if you become pregnant or you think you may be pregnant.
Tell all doctors, dentist and pharmacists who are treating you that you are taking CYTOTEC.
If an antacid is needed for stomach pain, use one which does not contain magnesium. Aluminium-containing antacids may be used when needed for relief of pain. Ask your doctor or pharmacist for advice if necessary.
Side effects
Check with your doctor as soon as possible if you have any problems while taking CYTOTEC even if you think they are not connected with the medicine or are not listed in this leaflet.
Like other medicines, CYTOTEC can cause some side effects. If they occur, they are most likely to be minor and temporary. However, some may be serious and need medical attention.
Stomach pains and diarrhoea are the most common side effects and are usually mild to moderate. If either of these effects occur, they usually settle down within a week or two. If you take CYTOTEC with food, you will have less chance of getting diarrhoea (or it will not be as bad, if you do get it). If you use an antacid (to reduce acid in your stomach), ask the pharmacist to recommend one which contains aluminium, since antacids which contain magnesium may make diarrhoea worse. Tell your doctor if stomach pains or diarrhoea are severe or do not stop after a week.
Other side effects include nausea, vomiting, flatulence (wind), indigestion, constipation, headaches, chills, fever or dizziness.
Occasionally women have menstrual problems.
Other side effects not listed above may occur in some patients. Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
After using CYTOTEC
Storage
Keep it where young children cannot reach it.
A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Do not store CYTOTEC or any other medicine in the bathroom or near a sink. Do not leave your tablets in the car or on windowsills.
Keep CYTOTEC in a cool dry place where the temperature stays below 25°C. Heat or moisture may cause CYTOTEC tablets to deteriorate.
Keep your tablets in their blister until it is time to take them. If you take the tablets out of the blister they may not keep well.
Disposal
If your doctor tells you to stop taking CYTOTEC, ask your pharmacist what to do with any tablets left over.
Product description
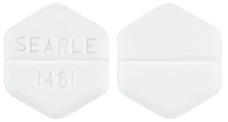
CYTOTEC tablets are scored, white, hexagonal tablets, marked on one side with SEARLE/1461.
Packs contain 120 tablets.
Ingredients
Each CYTOTEC tablet contains 200 micrograms of misoprostol as the active ingredient.
Other ingredients in each tablet are:
- cellulose-microcrystalline
- hypromellose
- sodium starch glycollate
- castor oil-hydrogenated
Supplier
CYTOTEC is supplied in Australia by:
Pfizer Australia Pty Ltd
Sydney NSW
Toll Free Number: 1800 675 229
www.pfizer.com.au
Australian Registration Number
AUST R 63983
This leaflet was revised in December 2019.
®Registered trademark
©Pfizer Australia Pty Ltd
Published by MIMS January 2020