1 Name of Medicine
Dasatinib.
2 Qualitative and Quantitative Composition
Dasatinib Sandoz film-coated tablets contain 20, 50, 70 or 100 mg of dasatinib.
Dasatinib is a white to light yellow colour powder.
Dasatinib Sandoz film-coated tablets contain sugars as lactose. For the full list of excipients, see Section 6.1 List of Excipients.
3 Pharmaceutical Form
Film-coated tablet.
The 20 mg tablets are white to off-white, round, biconvex, film-coated tablets, debossed with DAS on one side and 20 on the other side with approximate dimension of 5.6 mm.
The 50 mg tablets are white to off-white, oval, biconvex, film-coated tablets, debossed with DAS on one side and 50 on the other side with approximate dimension of 10.9 x 5.8 mm.
The 70 mg tablets are white to off-white, round, biconvex, film-coated tablets, debossed with DAS on one side and 70 on the other side with approximate dimension of 8.8 mm.
The 100 mg tablets are white to off-white, oval, biconvex, film-coated tablets, debossed with DAS on one side and 100 on the other side with approximate dimension of 14.9 x 7.2 mm.
4.1 Therapeutic Indications
Dasatinib Sandoz is indicated for the treatment of adults aged 18 years or over with:
Newly diagnosed Philadelphia chromosome positive (Ph+) chronic myeloid leukaemia in the chronic phase.
Chronic, accelerated or myeloid or lymphoid blast phase chronic myeloid leukaemia with resistance or intolerance to prior therapy including imatinib.
Newly diagnosed Philadelphia chromosome positive acute lymphoblastic leukaemia integrated with chemotherapy.
Philadelphia chromosome positive acute lymphoblastic leukaemia with resistance or intolerance to prior therapy.
Dasatinib Sandoz is indicated for the treatment of paediatric patients with:
Ph+ CML in the chronic phase.
Newly diagnosed Ph+ ALL in combination with chemotherapy.
4.2 Dose and Method of Administration
To achieve the recommended dose, Dasatinib Sandoz is available as 20 mg, 50 mg, 70 mg and 100 mg film-coated tablets. Dose increase or reduction is recommended based on patient response and tolerability.
Adult dosage.
CML.
The recommended starting dosage of Dasatinib Sandoz for chronic phase CML in adults is 100 mg administered orally once daily (QD). The recommended starting dosage of Dasatinib Sandoz for accelerated phase CML, myeloid or lymphoid blast phase CML in adults is 140 mg/day administered orally once daily and should be taken consistently either in the morning or the evening.
Ph+ ALL.
The recommended starting dosage of Dasatinib Sandoz for newly diagnosed Ph+ ALL in adults is 100 mg administered orally once daily (QD) or as clinically recommended. Dasatinib Sandoz should be taken consistently either in the morning or evening.
The recommended starting dosage of Dasatinib Sandoz for adult patients resistant or intolerant to prior therapy is 140 mg/day administered orally once daily (QD) and should be taken consistently either in the morning or the evening.
In clinical studies, treatment with dasatinib was continued in the maintenance phase until disease progression or until no longer tolerated by the patient.
Paediatric dosage.
Dosing for children is on the basis of body weight. Dasatinib is administered orally once daily in the form of Dasatinib Sandoz tablets (20 mg, 50 mg, 70 mg or 100 mg). Recalculate the dose every 3 months based on changes in body weight, or more often if necessary. Dasatinib Sandoz tablets are not recommended for patients weighing less than 10 kg.
There is no experience with dasatinib treatment in children under 1 year of age.
The recommended starting daily dosage of Dasatinib Sandoz tablets in paediatric patients is shown in Table 1.

Method of administration.
To be administered orally. Tablets must not be crushed, cut or chewed; they should be swallowed whole to maintain dosing consistency and minimize the risk of dermal exposure. Film-coated tablets should not be dispersed, as the exposure in patients receiving a dispersed tablet is lower than in those swallowing a whole tablet. However, there are additional administration considerations for paediatric patients who have difficulty swallowing tablets whole (see Section 4.4 Special Warnings and Precautions for Use, Paediatric use).
Dasatinib Sandoz can be taken with or without a meal and should be taken consistently either in the morning or the evening. Dasatinib Sandoz should not be taken with grapefruit or grapefruit juice (see Section 4.5 Interactions with Other Medicines and Other Forms of Interactions).
Treatment duration.
In clinical studies, treatment with dasatinib in adults with chronic phase CML; accelerated, myeloid or lymphoid blast phase (advanced phase) CML; or Ph+ ALL and in paediatric patients with chronic phase CML was continued until disease progression or until no longer tolerated by the patient. The effect of stopping treatment on long-term disease outcome after the achievement of a cytogenetic response (including complete cytogenetic response (CCyR) or major molecular response (MMR and MR4.5) has not been investigated.
In clinical studies, treatment with dasatinib in paediatric patients with Ph+ ALL was administered continuously, added to successive blocks of backbone chemotherapy, for a maximum duration of two years. In patients that receive a subsequent stem cell transplantation, dasatinib can be administered for an additional year post-transplantation.
Dose escalation.
In clinical studies in adult CML patients, dose escalation to 140 mg once daily (chronic phase CML) or 180 mg once daily (advanced phase CML) was allowed in patients who did not achieve a haematologic or cytogenetic response at the recommended starting dosage.
In clinical studies in adult Ph+ ALL patients, dose escalation to 140 mg daily (newly diagnosed patients) or 180 mg once daily (patients on prior therapy) was allowed in patients who did not achieve a haematologic or cytogenetic response at the recommended starting dosage.
The following dose escalations shown in Table 2 are recommended in paediatric patients with chronic phase CML who do not achieve a haematologic, cytogenic or molecular response at the recommended time points, per current treatment guidelines, and who tolerate treatment.
 Dose escalation is not recommended for paediatric patients with Ph+ ALL, as dasatinib is administered in combination with chemotherapy in these patients.
Dose escalation is not recommended for paediatric patients with Ph+ ALL, as dasatinib is administered in combination with chemotherapy in these patients.
Dose adjustment for adverse reactions.
Myelosuppression.
In clinical studies, myelosuppression was managed by dose interruption, dose reduction, or discontinuation of study therapy. Platelet transfusion and red cell transfusion were used as appropriate. Haematopoietic growth factor has been used in patients with resistant myelosuppression. Guidelines for dose modifications are summarised in Table 3 and Table 4.

 For paediatric patients with chronic phase CML, if Grade ≥ 3 neutropenia or thrombocytopenia recurs during complete haematologic response (CHR), Dasatinib Sandoz should be interrupted, and may be subsequently resumed at a reduced dose. Temporary dose reductions for intermediate degrees of cytopenia and disease response should be implemented as needed.
For paediatric patients with chronic phase CML, if Grade ≥ 3 neutropenia or thrombocytopenia recurs during complete haematologic response (CHR), Dasatinib Sandoz should be interrupted, and may be subsequently resumed at a reduced dose. Temporary dose reductions for intermediate degrees of cytopenia and disease response should be implemented as needed.
For paediatric patients with Ph+ ALL, no dose modification is recommended in cases of haematologic Grade 1 to 4 toxicities. If neutropenia and/or thrombocytopenia result in delay of the next block of treatment by more than 14 days, Dasatinib Sandoz should be interrupted and resumed at the same dose level once the next block of treatment is started. If neutropenia and/or thrombocytopenia persist and the next block of treatment is delayed another 7 days, a bone marrow assessment should be performed to assess cellularity and percentage of blasts. If marrow cellularity is < 10%, treatment with Dasatinib Sandoz should be interrupted until ANC > 500/microL (0.5 x 109/L), at which time treatment may be resumed at full dose. If marrow cellularity is > 10%, resumption of treatment with Dasatinib Sandoz may be considered.
Non-haematologic adverse reactions.
If a moderate (Grade 2) non-haematologic adverse reaction develops with Dasatinib Sandoz, treatment should be interrupted until the adverse reaction has resolved or returned to baseline. The same dose should be resumed if this is the first occurrence and the dose should be reduced if this is a recurrent adverse reaction. If a severe (Grade 3 or 4) non-haematologic adverse reaction develops with dasatinib use, treatment must be withheld until the event has resolved or improved. Thereafter, treatment can be resumed as appropriate at a reduced dose depending on the severity and recurrence of the event.
For adult patients with chronic phase CML who received 100 mg once daily, dose reduction to 80 mg once daily with further reduction from 80 mg once daily to 50 mg once daily, if needed, is recommended. For adult patients with advanced phase CML or Ph+ ALL who received 140 mg once daily, dose reduction to 100 mg once daily with further reduction from 100 mg once daily to 50 mg once daily, if needed, is recommended.
For paediatric patients with chronic phase CML who develop non-haematologic adverse reactions, the dose reduction recommendations for haematologic adverse reactions that are described above should be followed.
For paediatric patients with Ph+ ALL who develop non-haematologic adverse reactions, if needed, one level of dose reduction should be followed, according to the dose reduction recommendations for haematologic adverse reactions that are described above. With the exception of liver function test abnormalities, treatment should be interrupted for cases of Grade ≥ 3 non-haematologic adverse reactions in paediatric patients with Ph+ ALL and resumed at a reduced dose when resolved to Grade ≤ 1. For elevated direct bilirubin over 5 times the institutional upper limit of normal (ULN), treatment should be interrupted until improvement to baseline or Grade ≤ 1. For elevated AST/ALT over 15 times the institutional ULN, treatment should be interrupted until improvement to baseline or Grade < 1. If these liver function test abnormalities recur after re-initiation of treatment with Dasatinib Sandoz, the dose should be reduced.
Dose reduction for concomitant use of strong CYP3A4 inhibitors.
The concomitant use of strong CYP3A4 inhibitors and grapefruit juice with Dasatinib Sandoz should be avoided (see Section 4.5 Interactions with Other Medicines and Other Forms of Interactions). If possible, an alternative concomitant medication with no or minimal enzyme inhibition potential should be selected. If Dasatinib Sandoz must be administered with a strong CYP3A4 inhibitor, consider a dose decrease to:
40 mg daily for patients taking Dasatinib Sandoz 140 mg daily;
20 mg daily for patients taking Dasatinib Sandoz 100 mg daily;
20 mg daily for patients taking Dasatinib Sandoz 70 mg daily.
For patients taking Dasatinib Sandoz 60 mg or 40 mg daily, consider interrupting Dasatinib Sandoz until the inhibitor is discontinued. Allow a washout period of approximately 1 week after the inhibitor is stopped before reinitiating Dasatinib Sandoz.
These reduced doses of Dasatinib Sandoz are predicted to adjust the area under the curve (AUC) to the range observed without CYP3A4 inhibitors. However, clinical data are not available with these dose adjustments in patients receiving strong CYP3A4 inhibitors. If Dasatinib Sandoz is not tolerated after dose reduction, either discontinue the strong CYP3A4 inhibitor or stop Dasatinib Sandoz until the inhibitor is discontinued. Allow a washout period of approximately 1 week after the inhibitor is stopped before the Dasatinib Sandoz dose is increased.
Special populations.
Elderly population.
No clinically relevant age-related pharmacokinetic differences have been observed in these patients. No specific dose recommendation is necessary in the elderly (see Section 4.4 Special Warnings and Precautions for Use, Use in the elderly).
Hepatic impairment.
Patients with mild, moderate or severe hepatic impairment may receive the recommended starting dose. However, caution is recommended when Dasatinib Sandoz is administered to patients with hepatic impairment (see Section 4.4 Special Warnings and Precautions for Use, Use in hepatic impairment).
Renal impairment.
No clinical trials were conducted with dasatinib in patients with decreased renal function (the study in patients with newly diagnosed chronic phase CML excluded patients with serum creatinine > 3 times the upper limit of the normal range, and studies in patients with chronic phase CML with resistance or intolerance to prior imatinib therapy excluded patients with serum creatinine concentration > 1.5 times the upper limit of the normal range). Since the renal clearance of dasatinib and its metabolites is < 4%, a decrease in total body clearance is not expected in patients with renal insufficiency (see Section 4.4 Special Warnings and Precautions for Use, Use in renal impairment).4.3 Contraindications
Use of Dasatinib Sandoz is contraindicated in patients with hypersensitivity to dasatinib or to any other component of Dasatinib Sandoz.
4.4 Special Warnings and Precautions for Use
Myelosuppression.
Treatment with dasatinib is associated with thrombocytopenia, neutropenia and anaemia, which occur earlier and more frequently in patients with advanced phase CML or Ph+ ALL than in patients with chronic phase CML.
In adult patients with advanced phase CML or Ph+ ALL, complete blood counts (CBCs) should be performed weekly for the first 2 months and then monthly thereafter or as clinically indicated.
In adult and paediatric patients with chronic phase CML, CBCs should be performed every two weeks for 12 weeks, then every 3 months thereafter, or as clinically indicated.
In paediatric patients with Ph+ ALL treated in dasatinib in combination with chemotherapy, CBCs should be performed prior to the start of each block of chemotherapy and as clinically indicated. During the consolidation blocks of chemotherapy, CBCs should be performed every 2 days until recovery.
Myelosuppression is generally reversible and usually managed by withholding Dasatinib Sandoz temporarily or dose reduction (see Section 4.2 Dose and Method of Administration, Dose adjustment for adverse reactions; Section 4.4 Special Warnings and Precautions for Use, Effects on laboratory tests). CTC Grade 3 or 4 (severe) cases of anaemia were managed with blood transfusions.
Bleeding.
In the pooled population of Phase III studies in patients with chronic phase CML, 5 patients (1%) receiving dasatinib at the recommended dose 100 mg daily (n=548) had drug related Grade 3 or 4 haemorrhage. In the pooled population of clinical studies in patients with advanced phase CML or Ph+ ALL, severe (Grade 3 - 5) drug-related CNS haemorrhage, including fatalities, occurred in 1% of patients receiving dasatinib at the recommended dose 140 mg daily (n=304). Eight cases were fatal and 6 of them were associated with Common Toxicity Criteria (CTC) Grade 4 thrombocytopenia. Grade 3 or 4 drug-related gastrointestinal haemorrhage, including fatalities, occurred in 6% of patients and generally required treatment interruptions and transfusions. Other cases of Grade 3 or 4 drug-related haemorrhage occurred in 2% of patients treated with dasatinib 140 mg daily dose. Most bleeding reactions in clinical studies were typically associated with Grade 3 or 4 thrombocytopenia. Additionally, in vitro and in vivo platelet assays suggest that dasatinib treatment reversibly affects platelet activation.
Caution should be exercised if patients are required to take medications that inhibit platelet function or anticoagulants.
Fluid retention.
Dasatinib is associated with fluid retention. After 5 years of follow-up in the Phase III clinical study in patients with newly diagnosed chronic phase CML (n=258), drug-related Grade 3 or 4 fluid retention was reported in 13 patients (5%) receiving dasatinib compared to 2 patients (1%) receiving imatinib (n=258) (see Section 4.8 Adverse Effects (Undesirable Effects)). The cumulative incidence of drug-related pleural effusion (all Grades) in dasatinib-treated subjects increased over time; 7.8% new AEs of pleural effusion occurred in the first year of therapy followed by a smaller, yet consistent increase of ~5% of subjects/year after 24, 36, 48, and 60 months of treatment, respectively. The majority were low grade. In the pooled population of patients with chronic phase CML (n=548), severe (Grade 3 - 4) drug-related fluid retention occurred in 32 (6%) patients receiving dasatinib at the 100 mg once daily recommended dose.
In clinical studies in patients with advanced phase CML or Ph+ ALL receiving dasatinib at the approved dose 140 mg daily (n=304), Grade 3 or 4 drug-related fluid retention was reported in 8% of patients, including severe pleural and pericardial effusion reported in 7% and 1% of patients, respectively. Severe congestive heart failure/cardiac dysfunction was reported in 1% of patients. In these patients, severe pulmonary oedema and severe pulmonary hypertension were each reported in 1% of patients.
Patients who develop symptoms suggestive of pleural effusion or other fluid retention such as new or worsened dyspnoea on exertion or at rest, pleuritic chest pain, or dry cough should be evaluated promptly with chest X-ray or additional diagnostic imaging as appropriate. Fluid retention reactions were typically managed with dasatinib dose interruption or reduction and supportive care measures that may include diuretics or short courses of steroids. Severe pleural effusion may require thoracentesis and oxygen therapy. Dose modification should be considered. While the safety profile of dasatinib in the elderly population was similar to that in the younger population, patients aged 65 years and older are more likely to experience pleural effusion, congestive heart failure, gastrointestinal bleeding, and dyspnoea, and should be monitored closely.
Cases of chylothorax have also been reported in patients presenting with pleural effusion. Some cases of chylothorax resolved upon dasatinib discontinuation, interruption or dose reduction but most cases also required additional treatment (see Section 4.8 Adverse Effects (Undesirable Effects)).
QT prolongation.
In vitro data showing inhibition of the hERG K+ channel expressed in mammalian cells and action potential prolongation in rabbit Purkinje fibres by dasatinib and a number of its metabolites suggest that dasatinib has the potential to prolong cardiac ventricular repolarisation (QT interval).
After 5 years of follow-up in the Phase III study in newly diagnosed chronic phase CML, 1 patient (< 1%) in each of the dasatinib (n=258) and imatinib (n=258) treatment groups had QTc prolongation reported as an adverse reaction. The median changes in QTcF from baseline were 3.0 msec in dasatinib-treated patients compared to 8.2 msec in imatinib-treated patients. One patient (< 1%) in each group experienced a QTcF > 500 msec. In 865 patients with leukaemia treated with dasatinib in Phase II, single-arm clinical studies, the mean QTc interval changes from baseline using Fridericia's method (QTcF) were 4-6 msec; the upper 95% confidence intervals for all mean changes from baseline were < 7 msec. Of the 2,182 patients with resistance or intolerance to prior imatinib therapy treated with dasatinib, 15 (1%) had QT prolongation reported as an adverse reaction. Twenty-one (21) of these patients (1%) experienced a QTcF > 500 msec.
Dasatinib Sandoz should be administered with caution in patients who have or may develop prolongation of QTc. These include patients with hypokalaemia or hypomagnesaemia, patients with congenital long QT syndrome, patients taking anti-arrhythmic medicines or other medicinal products which lead to QT prolongation and cumulative high dose anthracycline therapy. Hypokalaemia or hypomagnesaemia should be corrected prior to Dasatinib Sandoz administration.
Cardiac adverse reactions.
Dasatinib was studied in a randomised trial of 519 patients with newly diagnosed CML in chronic phase which included patients with prior cardiac disease. The cardiac adverse reactions of congestive heart failure/cardiac dysfunction (1.9%), pericardial effusion (4.3%), arrhythmias (1.2%), palpitations (1.9%), QT prolongation (0.4%) and myocardial infarction (0.4%) (including fatal) were reported in patients taking dasatinib (n=258). Adverse cardiac reactions were more frequent in patients with risk factors or a previous medical history of cardiac disease. Patients with risk factors or a history of cardiac disease should be monitored carefully for signs or symptoms consistent with cardiac dysfunction and should be evaluated and treated appropriately.
Patients with uncontrolled or significant cardiovascular disease were not included in the clinical studies.
Pulmonary arterial hypertension.
Pulmonary arterial hypertension (PAH), confirmed by right heart catheterization, has been reported in association with dasatinib treatment. In these cases, PAH was reported after initiation of dasatinib therapy, including after more than one year of treatment. Patients with PAH reported during dasatinib treatment were often taking concomitant medications or had co-morbidities in addition to the underlying malignancy.
Patients should be evaluated for signs and symptoms of underlying cardiopulmonary disease prior to initiating Dasatinib Sandoz therapy. Patients who develop dyspnoea and fatigue after initiation of therapy should be evaluated for more common etiologies including pleural effusion, pulmonary oedema, anaemia, or lung infiltration. During this evaluation, guidelines for non-hematologic adverse reactions should be followed (see Section 4.2 Dose and Method of Administration, Dose adjustment for adverse reactions): if the adverse reaction is severe, treatment must be withheld until the event has resolved or improved. If no alternative diagnosis is found, the diagnosis of PAH should be considered. If PAH is confirmed, Dasatinib Sandoz should be permanently discontinued. Follow up should be performed according to standard practice guidelines. Improvements in hemodynamic and clinical parameters have been observed in dasatinib treated patients with PAH following cessation of dasatinib therapy.
Thrombotic microangiopathy (TMA).
BCR-ABL tyrosine kinase inhibitors (TKIs) have been associated with thrombotic microangiopathy (TMA), including individual case reports for dasatinib (see Section 4.8 Adverse Effects (Undesirable Effects)). If laboratory or clinical findings associated with TMA occur in a patient receiving Dasatinib Sandoz, treatment with Dasatinib Sandoz should be discontinued and thorough evaluation for TMA, including ADAMTS13 activity and anti-ADAMTS13-antibody determination, should be completed. If anti-ADAMTS13-antibody is elevated in conjunction with low ADAMTS13 activity, treatment with Dasatinib Sandoz should not be resumed.
Hepatitis B virus reactivation.
BCR-ABL TKIs have been associated with hepatitis B virus (HBV) reactivation including individual case reports for dasatinib. In some instances, HBV reactivation occurring in conjunction with other BCR-ABL TKIs resulted in acute hepatic failure or fulminant hepatitis leading to liver transplantation or a fatal outcome.
Screening for HBV should be considered in accordance with published guidelines before starting therapy with Dasatinib Sandoz. Consultation with a physician with expertise in the treatment of HBV is recommended for patients who test positive for HBV serology.
Patients who are carriers of HBV and require treatment with BCR-ABL TKIs should be closely monitored for clinical and laboratory signs of active HBV infection throughout therapy and for several months following termination of therapy. In patients who develop reactivation of HBV while receiving Dasatinib Sandoz, prompt consultation with a physician with expertise in the treatment of HBV is recommended.
Severe dermatologic reactions.
Individual cases of severe mucocutaneous dermatologic reactions, including Stevens-Johnson syndrome and erythema multiforme, have been reported with the use of dasatinib. Dasatinib Sandoz should be permanently discontinued in patients who experience a severe mucocutaneous reaction during treatment if no other etiology can be identified.
Lactose content.
Dasatinib Sandoz contains 135 mg of lactose monohydrate in a 100 mg daily dose and 189 mg of lactose monohydrate in a 140 mg daily dose.
Effect on growth and development in paediatric patients.
In paediatric trials of dasatinib in chronic phase CML imatinib-resistant/intolerant paediatric patients and treatment-naive paediatric patients after at least 2 years of treatment, treatment-related adverse events associated with bone growth and development were reported in 6 (4.6%) patients, one of which was severe in intensity (Growth Retardation Grade 3). These 6 cases included cases of epiphyses delayed fusion, osteopenia, growth retardation, and gynecomastia (see Section 5.1 Pharmacodynamic Properties). These results are difficult to interpret in the context of chronic diseases such as CML and require long-term follow-up.
Hepatotoxicity.
Dasatinib may cause hepatotoxicity as measured by elevations in bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (see Section 4.8 Adverse Effects (Undesirable Effects)). Monitor transaminases at baseline and monthly or as clinically indicated during treatment. Reduce dose, withhold, or permanently discontinue Dasatinib Sandoz based on severity. When dasatinib is administered in combination with chemotherapy, liver toxicity in the form of transaminase elevation and hyperbilirubinemia has been observed. Monitor hepatic function when Dasatinib Sandoz is used in combination with chemotherapy.
Use in hepatic impairment.
Based on the findings from a single-dose pharmacokinetic study, patients with mild, moderate or severe hepatic impairment may receive the recommended starting dose (see Section 4.2 Dose and Method of Administration, Special populations; Section 5.2 Pharmacokinetic Properties, Special populations). Due to the limitations of this clinical study, caution is recommended when Dasatinib Sandoz is administered to patients with hepatic impairment.
Use in renal impairment.
There are currently no clinical studies with dasatinib in patients with impaired renal function (the study in patients with newly diagnosed chronic phase CML excluded patients with serum creatinine > 3 times the upper limit of the normal range, and clinical studies in patients with chronic phase CML with resistance or intolerance to prior imatinib therapy have excluded patients with serum creatinine concentration > 1.5 times the upper limit of the normal range). Dasatinib and its metabolites are minimally excreted via the kidney. Since the renal excretion of unchanged dasatinib and its metabolites is < 4%, a decrease in total body clearance is not expected in patients with renal insufficiency.
Use in the elderly.
Of the 2,712 patients in clinical studies of dasatinib, 617 (23%) were 65 years of age and older and 123 (5%) were 75 years of age and older.
Patients aged 65 years and older are more likely to experience the commonly reported adverse reactions appetite disturbance (14.5% vs 8.0%), fatigue (27.4% vs 19.7%), pleural effusion (46.2 vs 28.4), cough (13.6% vs 8.4%), and dyspnoea (34.5% vs 17.7%)), and more likely to experience the less frequently reported adverse events abdominal distention (3.9% vs 2.9%), dizziness (7.1% vs 4.6%), lower gastrointestinal haemorrhage (2.4% vs 0.7%), pericardial effusion (7.6% vs 4.9%), congestive heart failure (3.1% vs 0.7%) and weight decrease (7.5% vs 3.7%), and should be monitored closely.
Comparisons based on efficacy are based on limited number of subjects in specific age groups in individual studies, however similar rates of cCCyR and MMR were observed between older and younger patients.
Paediatric use.
The safety and efficacy of dasatinib in paediatric patients for indications other than Ph+ chronic phase CML and Ph+ ALL (see Section 5.1 Pharmacodynamic Properties, Clinical trials) have not been established.
Paediatric patients with difficulty swallowing tablets.
Five patients with Ph+ ALL 2 to 10 years of age received at least one dose of dasatinib tablet dispersed in juice in Study CA180372. The exposure for dispersed tablets was 36% lower as compared to intact tablets in paediatric patients (see Section 5.2 Pharmacokinetic Properties, Special populations, Paediatric patients). Due to the limited available clinical data, it is unclear whether dispersing dasatinib tablets significantly alters the safety and/or efficacy of dasatinib.
Effects on laboratory tests.
Haematology and biochemistry in patients with newly diagnosed chronic phase CML.
The comparative frequency of Grade 3 and 4 laboratory abnormalities in patients with newly diagnosed chronic phase CML is presented in Table 5. There were no discontinuations of dasatinib therapy due to the biochemical laboratory parameters.

Haematology and biochemistry in patients with resistance or intolerance to prior imatinib therapy.
Table 6 shows laboratory findings from clinical trials in CML patients with imatinib resistance or intolerance received at 24 months of follow up.
 Myelosuppression was commonly reported in all patient populations. In newly diagnosed chronic phase CML, myelosupression was less frequently reported than in chronic phase CML patients with resistance or intolerance to prior imatinib therapy. The frequency of Grade 3 or 4 neutropenia, thrombocytopenia, and anaemia was higher in patients with advanced CML or Ph+ ALL than in chronic phase CML.
Myelosuppression was commonly reported in all patient populations. In newly diagnosed chronic phase CML, myelosupression was less frequently reported than in chronic phase CML patients with resistance or intolerance to prior imatinib therapy. The frequency of Grade 3 or 4 neutropenia, thrombocytopenia, and anaemia was higher in patients with advanced CML or Ph+ ALL than in chronic phase CML.
In patients who experienced Grade 3 or 4 myelosuppression, recovery generally occurred following dose interruption or reduction; permanent discontinuation of treatment occurred in 2% of newly diagnosed chronic phase CML patients in the Phase III study and in 5% of patients with resistance or intolerance to prior imatinib therapy in the Phase III study.
Grade 3 or 4 elevations in transaminases or bilirubin and Grade 3 or 4 hypocalcaemia, hypokalaemia, and hypophosphataemia were reported in all phases of CML but were reported with an increased frequency in patients with myeloid or lymphoid blast phase CML and Ph+ ALL. Elevations in transaminases or bilirubin were usually managed with dose reduction or interruption. In general, decreased calcium levels were not associated with clinical symptoms. Patients developing Grade 3 or 4 hypocalcaemia often had recovery with oral calcium supplementation.
In the paediatric CML studies, the rates of laboratory abnormalities were consistent with the known profile for laboratory parameters in adults. In the paediatric ALL studies, the rates of laboratory abnormalities were consistent with the known safety profile in adults, within the context of an acute leukaemia patients receiving a background chemotherapy regimen.4.5 Interactions with Other Medicines and Other Forms of Interactions
Drugs that may increase dasatinib plasma concentrations.
CYP3A4 inhibitors.
In vitro, dasatinib is a CYP3A4 substrate. Concomitant use of Dasatinib Sandoz and substances that potently inhibit CYP3A4 (e.g. ketoconazole, itraconazole, erythromycin, clarithromycin, ritonavir, atazanavir, lopinavir, grapefruit juice) may increase exposure to dasatinib. Therefore, in patients receiving treatment with Dasatinib Sandoz, systemic administration of a potent CYP3A4 inhibitor is not recommended. Selection of an alternate concomitant medication with no or minimal CYP3A4 inhibition potential is recommended. If systemic administration of a potent CYP3A4 inhibitor cannot be avoided, the patient should be closely monitored for toxicity (see Section 4.2 Dose and Method of Administration).
Drugs that may decrease dasatinib plasma concentrations.
CYP3A4 inducers.
Drugs that induce CYP3A4 activity may increase metabolism and decrease dasatinib plasma concentration. Therefore, concomitant use of potent CYP3A4 inducers (e.g. phenytoin, carbamazepine, rifampicin, phenobarbital or Hypericum perforatum, also known as St. John's Wort) with Dasatinib Sandoz is not recommended. In healthy subjects, the concomitant use of dasatinib and rifampicin, a potent CYP3A4 inducer, resulted in a five-fold decrease in dasatinib exposure. In patients for whom rifampicin or other CYP3A4 inducers are indicated, alternative agents with less enzyme induction potential should be used. Concomitant use of dexamethasone, a weak CYP3A4 inducer, with dasatinib is allowed; dasatinib AUC is predicted to decrease approximately 25% with concomitant use of dexamethasone, which is not likely to be clinically meaningful.
Antacids.
Non-clinical data demonstrate that the solubility of dasatinib is pH dependent. In healthy subjects, the concomitant use of aluminium hydroxide/magnesium hydroxide antacids with dasatinib reduced the AUC of a single dose of dasatinib by 55% and the Cmax by 58%. However, when antacids were administered 2 hours prior to a single dose of dasatinib, no relevant changes in dasatinib, concentration or exposure were observed. Thus, antacids may be administered up to 2 hours prior to or 2 hours following Dasatinib Sandoz. Simultaneous administration of Dasatinib Sandoz with antacids should be avoided.
Histamine-2 antagonists/proton pump inhibitors.
Long-term suppression of gastric secretion by histamine-2 antagonists or proton pump inhibitors (e.g. famotidine and omeprazole) is likely to reduce dasatinib exposure. The concomitant use of histamine-2 antagonists or proton pump inhibitors with Dasatinib Sandoz is not recommended. In a single-dose study in healthy subjects, the administration of famotidine 10 hours prior to a single dose of dasatinib reduced dasatinib exposure by 61%. The use of antacids should be considered in place of histamine-2 antagonists or proton pump inhibitors in patients receiving Dasatinib Sandoz therapy.
Drugs that may have their plasma concentration altered by dasatinib.
CYP3A4 substrates.
In a study in healthy subjects, a single 100 mg dose of dasatinib increased exposure to simvastatin, a known CYP3A4 substrate, by 20%. Therefore, CYP3A4 substrates known to have a narrow therapeutic index such as astemizole, terfenadine, cisapride, pimozide, quinidine, bepridil or ergot alkaloids (ergotamine, dihydroergotamine) should be administered with caution in patients receiving Dasatinib Sandoz (see Section 5.1 Pharmacodynamic Properties, Mechanism of action).
Other interactions.
In vitro data indicate a potential risk for interaction with CYP2C8 substrates, such as glitazones. No specific drug interaction studies between dasatinib and chemotherapy regimens routinely used in newly diagnosed Ph+ ALL patients have been performed.4.6 Fertility, Pregnancy and Lactation
Effects on fertility.
Dasatinib did not affect male or female fertility in a conventional rat fertility and early embryonic development study at approximately 1x human clinical exposure (100 or 140 mg dose). However, embryolethality was evident when dams were treated at these doses. Dasatinib caused atrophy/degeneration of the testis in rats and monkeys and an increase in the number of corpora lutea in the ovaries in rats at doses producing plasma exposure levels below or close to that anticipated in patients receiving Dasatinib Sandoz therapy. Data evaluating reproductive toxicity in male patients taking dasatinib is limited (see Section 4.6 Fertility, Pregnancy and Lactation, Use in pregnancy).
(Category D)
Dasatinib may cause fetal harm when administered to a pregnant woman (see Section 4.6 Fertility, Pregnancy and Lactation, Embryofetal toxicity). In non-clinical studies, at exposure levels that are readily achievable in humans receiving therapeutic doses of 100 mg of dasatinib serious embryo fetal toxicity was observed in both pregnant rats and rabbits. Malformations (including skeletal alterations) and fetal death were observed in rats treated with dasatinib.
Dasatinib Sandoz is therefore not recommended for use in women who are pregnant or contemplating pregnancy. Women must be advised to avoid becoming pregnant while on therapy. If Dasatinib Sandoz is used during pregnancy, or if the patient becomes pregnant while taking Dasatinib Sandoz, the patient should be apprised of the potential hazard to the fetus.
The potential effects of dasatinib on sperm have been evaluated in an oral study of fertility and early embryonic development in rats. Dasatinib is not a reproductive toxicant in male rats at clinically relevant exposures (see Section 4.6 Fertility, Pregnancy and Lactation, Effects on fertility). However, data evaluating reproductive toxicity in male patients taking dasatinib are limited.
Sexually active male or female patients of childbearing potential taking Dasatinib Sandoz should use adequate contraception.
Embryofetal toxicity.
Dasatinib can cause fetal harm when administered to a pregnant woman. There have been post-marketing reports of spontaneous abortion and fetal and infant anomalies from women who have taken dasatinib during pregnancy.
It is unknown whether Dasatinib Sandoz is excreted in human milk. Women who are taking Dasatinib Sandoz should not breastfeed. In an exploratory peri- and postnatal development study in rats, dasatinib was detectable in the plasma of breast-fed pups with levels 30-40% of the maternal levels. Pleural effusion and deaths were seen in maternally exposed rat pups, indicating indirect exposure of dasatinib was incompatible with pup survival, even at sub-therapeutic maternal exposure.4.7 Effects on Ability to Drive and Use Machines
The effects of this medicine on a person's ability to drive and use machines were not assessed as part of its registration.
4.8 Adverse Effects (Undesirable Effects)
Dasatinib as a single-agent therapy.
In total, the clinical trial experience for dasatinib administered as single-agent therapy represents 2809 patients, of which 2712 were adult and 97 paediatric. In the 2712 adult patients with either chronic phase CML, advanced phase CML or Ph+ ALL, the median duration of therapy was 19.2 months (range 0 to 93.2 months). In the subset of 97 paediatric patients with chronic phase CML, the median duration of therapy was 51.1 months (range 1.9 to 99.6 months).
The majority of patients treated with dasatinib, regardless of dose or schedule, experienced adverse reactions at some time. In the overall population of 2712 dasatinib-treated adult subjects, 520 (19%) experience adverse drug reactions leading to treatment discontinuation.
The overall safety profile of dasatinib in the paediatric population was similar to that of the adult population, with the exception of no reported pericardial effusion, pleural effusion, pulmonary oedema, or pulmonary hypertension in the paediatric population. Of the 97 paediatric patients with chronic phase CML, 1 (1%) experienced adverse reactions leading to treatment discontinuation.
No dedicated clinical trials have been conducted investigating the safety of dasatinib in newly diagnosed Ph+ ALL as a primary outcome. The safety profile of dasatinib in 4 Phase II clinical trials in newly diagnosed patients with Ph+ALL, was generally consistent with the safety profile in patients who were resistant or intolerant to prior therapy.
The following adverse reactions, excluding laboratory abnormalities, were reported in patients in clinical trials where dasatinib was administered as single-agent therapy. These reactions are presented by system organ class and by frequency. Frequencies are defined as: very common (≥ 1/10); common (≥ 1/100 to < 1/10); uncommon (≥ 1/1,000 to < 1/100); rare (≥ 1/10,000 to < 1/1,000). Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
Investigations.
Common: weight decreased, weight increased.
Uncommon: blood creatine phosphokinase increased, gamma-glutamyl transferase increased.
Cardiac disorders.
Common: congestive heart failure/cardiac dysfunctionc, pericardial effusion, arrhythmia (including tachycardia), palpitations.
Uncommon: myocardial infarction (including fatal outcomes), electrocardiogram QT prolonged, pericarditis, ventricular arrhythmia (including ventricular tachycardia), angina pectoris, cardiomegaly, electrocardiogram T wave abnormal, troponin increased.
Rare: cor pulmonale, myocarditis, acute coronary syndrome, cardiac arrest, electrocardiogram PR prolongation, coronary artery disease, pleuropericarditis.
Blood and lymphatic system disorders.
Very common: myelosuppression (including anaemia, neutropenia, thrombocytopenia).
Common: febrile neutropenia.
Uncommon: lymphadenopathy, lymphopenia.
Rare: aplasia pure red cell.
Nervous system disorders.
Very common: headache.
Common: neuropathy (including peripheral neuropathy), dizziness, dysgeusia, somnolence.
Uncommon: CNS bleedingb, syncope, tremor, amnesia, balance disorder.
Rare: cerebrovascular accident, transient ischemic attack, convulsion, optic neuritis, VIIth nerve paralysis, dementia, ataxia.
Eye disorders.
Common: visual disorder (including visual disturbance, vision blurred, and visual acuity reduced), dry eye.
Uncommon: conjunctivitis, visual impairment, photophobia, lacrimation increased.
Ear and labyrinth disorders.
Common: tinnitus.
Uncommon: hearing loss, vertigo.
Respiratory, thoracic and mediastinal disorders.
Very common: pleural effusion, dyspnoea.
Common: pulmonary oedema, pulmonary hypertension, lung infiltration, pneumonitis, cough.
Uncommon: bronchospasm, asthma, dysphonia, pulmonary arterial hypertension.
Rare: acute respiratory distress syndrome, pulmonary embolism.
Gastrointestinal disorders.
Very common: diarrhoea, vomiting, nausea, abdominal pain.
Common: gastrointestinal bleeding (including fatal), colitis (including neutropenic colitis), gastritis, mucosal inflammation (including mucositis/stomatitis), dyspepsia, abdominal distension, constipation, oral soft tissue disorder.
Uncommon: pancreatitis, upper gastrointestinal ulcer, oesophagitis, ascites, anal fissure, dysphagia, gastro-oesophageal reflux disease.
Rare: protein-losing gastroenteropathy, ileus, pancreatitis acute, anal fistula.
Renal and urinary disorders.
Uncommon: renal failure, urinary frequency, proteinuria.
Rare: renal impairment.
Skin and subcutaneous tissue disorders.
Very common: skin rashc.
Common: alopecia, dermatitis (including eczema), pruritus, acne, dry skin, urticaria, hyperhidrosis.
Uncommon: neutrophilic dermatosis, photosensitivity, pigmentation disorder, panniculitis, skin ulcer, bullous conditions, nail disorder, palmar-plantar erythrodysesthesia syndrome, hair disorder.
Rare: leukocytoclastic vasculitis, skin fibrosis.
Musculoskeletal and connective tissue disorders.
Very common: musculoskeletal pain.
Common: arthralgia, myalgia, muscular weakness, musculoskeletal stiffness, muscle spasm.
Uncommon: rhabdomyolysis, tendonitis, muscle inflammation, osteonecrosis, arthritis.
Rare: epiphyses delayed fusiong, growth retardationg.
Metabolism and nutrition disorders.
Common: appetite disturbancesa, hyperuricaemia.
Uncommon: dehydration, hypoalbuminaemia, hypercholesterolemia, tumour lysis syndrome.
Rare: diabetes mellitus.
Infections and infestations.
Very common: infection (including bacterial, viral, fungal, non-specified).
Common: pneumonia (including bacterial, viral, and fungal), upper respiratory tract infection/inflammation, herpes virus infection, enterocolitis infection, sepsis (including uncommon reports of fatal outcome).
Injury, poisoning, and procedural complications.
Common: contusion.
Vascular disorders.
Very common: haemorrhaged.
Common: hypertension, flushing.
Uncommon: hypotension, thrombophlebitis, thrombosis.
Rare: deep vein thrombosis, embolism, livedo reticularis.
Not known: thrombotic microangiopathy.
General disorders and administration site conditions.
Very common: peripheral oedemag, fatigue, face oedemah, pyrexia.
Common: asthenia, pain, chest pain, generalised oedemai, chills.
Uncommon: malaise, other superficial oedemaj.
Rare: gait disturbance.
Immune system disorders.
Uncommon: hypersensitivity (including erythema nodosum).
Rare: Anaphylactic shockk.
Endocrine disorders.
Uncommon: hypothyroidism.
Rare: hyperthyroidism, thyroiditis.
Hepatobiliary disorders.
Uncommon: hepatitis, cholecystitis, cholestasis.
Reproductive system and breast disorders.
Uncommon: gynecomastia, menstrual disorder.
Pregnancy, puerperium and perinatal conditions.
Rare: abortion.
Psychiatric disorders.
Common: depression, insomnia.
Uncommon: anxiety, confusional state, affect lability, libido decreased.
a Includes decreased appetite, early satiety, increased appetite.
b Includes central nervous system haemorrhage, cerebral haematoma, cerebral haemorrhage, extradural haematoma, haemorrhage intracranial, haemorrhagic stroke, subarachnoid haemorrhage, subdural haematoma, and subdural haemorrhage.
c Includes brain natriuretic peptide increased, ventricular dysfunction, left ventricular dysfunction, right ventricular dysfunction, cardiac failure, cardiac failure acute, cardiac failure chronic, cardiac failure congestive, cardiomyopathy, congestive cardiomyopathy, diastolic dysfunction, ejection fraction decreased, ventricular failure, left ventricular failure, right ventricular failure, and ventricular hypokinesis.
d Excludes gastrointestinal bleeding and CNS bleeding; these ADRs are reported under the gastrointestinal disorders system organ class and the nervous system disorders system organ class, respectively.
e Includes drug eruption, erythema, erythema multiforme, erythrosis, exfoliative rash, generalised erythema, genital rash, heat rash, milia, miliaria, pustular psoriasis, rash, rash erythematous, rash follicular, rash generalised, rash macular, rash maculopapular, rash papular, rash pruritic, rash pustular, rash vesicular, skin exfoliation, skin irritation, toxic skin eruption, urticaria vesiculosa, and vasculitic rash.
f Reported only in paediatric studies. Frequency reported as common in paediatric studies vs rare in overall monotherapy population.
g Includes gravitational oedema, localised oedema, oedema peripheral.
h Includes conjunctival oedema, eye oedema, eye swelling, eyelid oedema, face oedema, lip oedema, macular oedema, oedema mouth, orbital oedema, periorbital oedema, swelling face.
i Includes fluid overload, fluid retention, gastrointestinal oedema, generalised oedema, peripheral swelling (reported only in paediatric studies), oedema, oedema due to cardiac disease, perinephric effusion, post procedural oedema, visceral oedema.
j Includes genital swelling, incision site oedema, oedema genital, penile oedema, penile swelling, scrotal oedema, skin swelling, testicular swelling, vulvovaginal swelling.
k Reported only in paediatric studies.
Dasatinib in combination with chemotherapy.
Paediatric patients with Ph+ ALL.
In Study CA180-372, 82 paediatric patients received dasatinib in combination with chemotherapy on a continuous dosing regimen. The median duration of therapy was 23.6 months (range 2.4 to 27.1 months). Of the 82 Ph+ ALL paediatric patients, 2 (2.4%) experienced adverse reactions leading to treatment discontinuation. Adverse reactions reported in Study CA180372 at a frequency of ≥ 10% are shown in Table 7.

Post-marketing experience.
The following additional adverse events have been identified during post approval use of dasatinib. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Infections and infestations.
Hepatitis B reactivation.
Cardiac disorders.
Atrial fibrillation/atrial fluttera.
Respiratory, thoracic and mediastinal disorders.
Interstitial lung disease, pleural effusion, chylothorax.
Skin and subcutaneous tissue disorders.
Stevens-Johnson syndromeb.
Renal and urinary disorders.
Nephrotic syndrome.
Hepatobiliary disorders.
Hepatotoxicity.
a Typically reported in elderly patients or in patients with confounding factors including significant underlying or concurrent cardiac or cardiovascular disorders, or other significant comorbidities (e.g. severe infection/sepsis, electrolyte abnormalities).
b In the post-marketing setting, individual cases of Stevens-Johnson syndrome have been reported. It could not be determined whether these mucocutaneous adverse reactions were directly related to dasatinib or to concomitant medications.
Reporting suspected adverse effects.
Reporting suspected adverse reactions after registration of the medicinal product is important. It allows continued monitoring of the benefit-risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions at www.tga.gov.au/reporting-problems.4.9 Overdose
Experience with overdose of dasatinib in clinical studies is limited to isolated cases. The highest overdosage of 280 mg per day for one week was reported in two patients and both developed a significant decrease in platelet counts. Since dasatinib is associated with severe myelosuppression, patients who ingest more than the recommended dosage should be closely monitored for myelosuppression and given appropriate supportive treatment.
For information on the management of overdose, contact the Poisons Information Centre on 131 126 (Australia).
5 Pharmacological Properties
5.1 Pharmacodynamic Properties
Mechanism of action.
Dasatinib is a potent inhibitor of multiple oncogenic kinases, cellular enzymes involved in the transmission of growth signals from the cell membrane to the nucleus. Dasatinib inhibits the activity of the BCR-ABL kinase and SRC-family kinases at low nanomolar or subnanomolar concentrations. Dasatinib also inhibits a number of other kinases including c-KIT, the EPHA2 receptor and the PDGFβ receptor. Unlike imatinib, it binds not only to the inactive but also to the active conformation of the BCR-ABL kinase. This suggests a reduced propensity for acquired drug resistance due to the emergence of mutations that promote the adoption of kinase's active conformation.
Dasatinib has been demonstrated to inhibit the survival/proliferation of human leukaemic cell lines in vitro, and to inhibit the growth of human CML (chronic myeloid leukaemia) xenografts in SCID mice, in both imatinib-sensitive and resistant models of the disease. Antileukaemic activity was seen in dasatinib-treated mice in a model of CML with CNS involvement. Non-clinical studies show that dasatinib can overcome imatinib resistance resulting from BCR-ABL independence, most BCR-ABL kinase domain mutations, activation of alternate signaling pathways involving SRC-family kinases (LYN and FYN) and P-glycoprotein (multi-drug resistance protein 1) overexpression.
Clinical trials.
In the Phase I study, haematologic and cytogenetic responses were observed in all phases of CML and in Ph+ ALL in the first 84 patients treated and followed for up to 27 months. Responses were durable across all phases of CML and Ph+ ALL.
Four single-arm, uncontrolled, open-label Phase II clinical trials were conducted to determine the safety and efficacy of dasatinib in patients with CML in chronic, accelerated, or myeloid blast phase, who were either resistant or intolerant to imatinib.
One randomized, comparative trial was conducted in chronic phase patients who failed initial treatment with 400 or 600 mg imatinib. The starting dose of dasatinib was 70 mg twice daily. Dose modifications were allowed for improving activity or management of toxicity.
Two randomised, open-label Phase III trials were conducted to evaluate the efficacy of dasatinib administered once daily compared with dasatinib administered twice daily. In addition, one open-label, randomised, comparative Phase III study was conducted in adult patients with newly diagnosed chronic phase CML.
The efficacy of dasatinib is based on haematological and cytogenetic response rates. Durability of response and estimated survival rates provide additional evidence of dasatinib clinical benefit.
A total of 2,712 patients were evaluated in clinical trials of CML; of these 23% were ≥ 65 years of age and 5% were ≥ 75 years of age.
Chronic phase CML - newly diagnosed adults.
An international open-label, multi-centre, randomised, comparative Phase III study was conducted in adult patients with newly diagnosed chronic phase CML. Patients were randomised to receive either dasatinib 100 mg once daily or imatinib 400 mg once daily. The primary end-point was the rate of confirmed complete cytogenetic response (cCCyR) within 12 months. Secondary endpoints included time in cCCyR (measure of durability of response), time to cCCyR, major molecular response (MMR) rate, time to MMR, progression free survival (PFS) and overall survival (OS). Other relevant efficacy results included CCyR and complete molecular response (CMR) rates.
A total of 519 patients were randomised to a treatment group: 259 to dasatinib and 260 to imatinib. Baseline characteristics were well balanced between the two treatment groups with respect to age (median age was 46 years for the dasatinib group and 49 years for the imatinib group with 10% and 11% of patients 65 years of age or older, respectively), gender (women 44% and 37%, respectively), and race (Caucasian 51% and 55%; Asian 42% and 37%, respectively). At baseline, the distribution of Hasford Scores was similar in the dasatinib and imatinib treatment groups (low risk: 33% and 34%; intermediate risk 48% and 47%; high risk: 19% and 19%, respectively).
With a minimum of 12 months follow-up, 85% of patients randomised to the dasatinib group and 81% of patients randomised to the imatinib group were still receiving first-line treatment. Discontinuation due to disease progression occurred in 3% of dasatinib-treated patients and 5% of imatinib-treated patients. With a minimum of 60 months follow-up, 61% of patients randomised to the dasatinib group and 63% of patients randomised to the imatinib group were still receiving first-line treatment. Discontinuation due to disease progression occurred in 7% of dasatinib-treated patients and 9% of imatinib-treated patients.
Efficacy results are presented in Table 8. A statistically significantly greater proportion of patients in the dasatinib group achieved a cCCyR compared with patients in the imatinib group within the first 12 months of treatment. Efficacy of dasatinib was consistently demonstrated across different subgroups, including age, gender, and baseline Hasford score.
 For time-to cCCyR, a hazard ratio of 1.55 indicates that a patient treated with dasatinib is 55% more likely to achieve a cCCyR at any time compared to a patient treated with imatinib. Similarly, for time-to MMR, a hazard ratio of 2.01 indicates a patient treated with dasatinib is more than two times more likely to achieve a MMR at any time compared to a patient treated with imatinib. For durability of cCCyR (time-in response), a hazard ratio of 0.7 indicates a patient treated with dasatinib is 30% less likely to have disease progression after achieving a cCCyR (or never achieving a cCCyR) compared to a patient treated with imatinib.
For time-to cCCyR, a hazard ratio of 1.55 indicates that a patient treated with dasatinib is 55% more likely to achieve a cCCyR at any time compared to a patient treated with imatinib. Similarly, for time-to MMR, a hazard ratio of 2.01 indicates a patient treated with dasatinib is more than two times more likely to achieve a MMR at any time compared to a patient treated with imatinib. For durability of cCCyR (time-in response), a hazard ratio of 0.7 indicates a patient treated with dasatinib is 30% less likely to have disease progression after achieving a cCCyR (or never achieving a cCCyR) compared to a patient treated with imatinib.
After 60 months follow-up, median time to cCCyR was 3.1 months in the 214 dasatinib group responders and 5.8 months in the 204 imatinib group responders. Median time to MMR after 60 months follow-up was 9.3 months in the 196 dasatinib group responders and 15.0 months in the 163 imatinib group responders. The rates of cCCyR in the dasatinib and imatinib treatment groups, respectively, within 3 months (54% and 30%), 6 months (70% and 56%), 9 months (75% and 63%), 24 months (80% and 74%), 36 months (83% and 77%), 48 months (83% and 79%) and 60 months (83% and 79%) were consistent with the primary endpoint. The rates of MMR in the dasatinib and imatinib treatment groups, respectively, within 3 months (8% and 0.4%), 6 months (27% and 8%), 9 months (39% and 18%), 12 months (46% and 28%), 24 months (64% and 46%), 36 months (67% and 55%), 48 months (73% and 60%) and 60 months (76% and 64%) were also consistent with the primary endpoint. With a minimum of 60 months follow up, the rate of CMR (i.e. at least 4.5-log reduction from a standardised baseline value BCR-ABL ratio ≤ 0.0032%) at any time was 44% versus 34% in the dasatinib and imatinib treatment groups, respectively.
In an exploratory subgroup analysis, the rate of MMR at any time in each risk group determined by Hasford score was higher in the dasatinib group compared with the imatinib group (low risk: 90% and 69%; intermediate risk: 71% and 65%; high risk: 67% and 54%, respectively).
In an exploratory analysis, more dasatinib-treated subjects (84%) achieved early molecular response (defined as BCR-ABL levels ≤ 10% at 3 months) compared with imatinib-treated subjects (64%). Subjects achieving early molecular response had a lower risk of transformation, higher rate of progression-free survival (PFS) and higher rate of overall survival (OS), as shown in Table 9 and Table 10.

 The progression-free survival rate by specific timepoint is displayed graphically in Figure 1. Rate of PFS was consistently higher in dasatinib-treated patients who achieved BCR-ABL level ≤ 10 percent at 3 months than those who did not.
The progression-free survival rate by specific timepoint is displayed graphically in Figure 1. Rate of PFS was consistently higher in dasatinib-treated patients who achieved BCR-ABL level ≤ 10 percent at 3 months than those who did not.
 The overall survival rate by specific timepoint is displayed graphically in Figure 2. Rate of OS was consistently higher in dasatinib-treated patients who achieved BCR-ABL level ≤ 10 percent at 3 months than those who did not.
The overall survival rate by specific timepoint is displayed graphically in Figure 2. Rate of OS was consistently higher in dasatinib-treated patients who achieved BCR-ABL level ≤ 10 percent at 3 months than those who did not.
 The time to MMR is displayed graphically in Figure 3. The time to MMR was consistently shorter in dasatinib-treated subjects compared with imatinib-treated subjects.
The time to MMR is displayed graphically in Figure 3. The time to MMR was consistently shorter in dasatinib-treated subjects compared with imatinib-treated subjects.
 MMR rates by specific timepoint are displayed graphically in Figure 4. Rates of MMR were consistently higher in dasatinib-treated subjects compared with imatinib-treated subjects.
MMR rates by specific timepoint are displayed graphically in Figure 4. Rates of MMR were consistently higher in dasatinib-treated subjects compared with imatinib-treated subjects.
 MR4.5 rates over time are displayed graphically in Figure 5. Rate of MR4.5 over time was consistently higher in dasatinib-treated subjects compared with imatinib-treated subjects.
MR4.5 rates over time are displayed graphically in Figure 5. Rate of MR4.5 over time was consistently higher in dasatinib-treated subjects compared with imatinib-treated subjects.
 Disease progression was defined as increasing white blood cells despite appropriate therapeutic management, loss of CHR (complete haematologic response), partial CyR or CCyR, progression to accelerated phase or blast phase, or death. The estimated 60-month PFS rate was 88.9% (CI: 84.0% - 92.4%) and 89.2% (CI: 84.3% - 92.7%) for the dasatinib and imatinib treatment groups, respectively. Transformation to accelerated or blast phase occurred less frequently with dasatinib (n = 8; 3.1%) than with imatinib-treated patients (n = 15; 5.8%). The estimated 60-month survival rates for dasatinib and imatinib-treated patients were 90.9% (CI: 86.6% - 93.8%) and 89.6% (CI: 85.2% - 92.8%) respectively.
Disease progression was defined as increasing white blood cells despite appropriate therapeutic management, loss of CHR (complete haematologic response), partial CyR or CCyR, progression to accelerated phase or blast phase, or death. The estimated 60-month PFS rate was 88.9% (CI: 84.0% - 92.4%) and 89.2% (CI: 84.3% - 92.7%) for the dasatinib and imatinib treatment groups, respectively. Transformation to accelerated or blast phase occurred less frequently with dasatinib (n = 8; 3.1%) than with imatinib-treated patients (n = 15; 5.8%). The estimated 60-month survival rates for dasatinib and imatinib-treated patients were 90.9% (CI: 86.6% - 93.8%) and 89.6% (CI: 85.2% - 92.8%) respectively.
In a phase III trial of newly diagnosed chronic phase CML, BCR-ABL sequencing was performed on blood samples from patients who discontinued dasatinib or imatinib therapy. Among dasatinib-treated patients the mutations detected were T315I, F317I/L and V299L. Dasatinib does not appear to be active against the T315I mutation, based on in vitro data.
Phase III clinical trials in patients with CML in chronic, accelerated, or myeloid blast phase, and Ph+ ALL who were resistant or intolerant to imatinib.
Two randomised, open-label studies were conducted to evaluate the efficacy of dasatinib administered once daily compared with dasatinib administered twice daily: The results described in Table 11 and Table 12 are based on a minimum of 24 months and 60 months follow-up after the start of dasatinib therapy.
In the non-inferiority study in chronic phase CML, the primary endpoint was MCyR (once daily vs. twice daily) in imatinib-resistant patients. The main secondary endpoint was MCyR by total daily dose level in the imatinib-resistant patients. Other secondary endpoints included duration of MCyR, progression-free survival, and overall survival. A total of 670 patients, of whom 497 were imatinib-resistant, were randomised to the dasatinib 100 mg once daily, 140 mg once daily, 50 mg twice daily, or 70 mg twice daily group. The non-inferiority criteria were met for the primary efficacy endpoint at 6 months analysis. Results for each of the 4 individual regimens demonstrated comparable efficacy for MCyR and for a variety of secondary endpoints. At 2-year analysis, the median duration of treatment was approximately 22 months (range < 1 to 31 months). In patients with resistant or intolerant chronic phase CML, the median duration of treatment for patients still on therapy (n=205) was 59 months (range 28 to 66 months).
Efficacy results at 2 year analysis are presented in Table 11 and Table 12. Efficacy was achieved across all dasatinib treatment groups with the once daily schedule demonstrating comparable efficacy (non-inferiority) to the twice daily schedule on the primary efficacy end-point (difference in MCyR 1.9%; 95% confidence interval [-6.8% - 10.6%]). However, the 100 mg once daily regimen had improved tolerability.
 Efficacy was also assessed in patients who were intolerant to imatinib. In this population of patients who received 100 mg once daily, MCyR was achieved in 77% and CCyR in 67% with a minimum of 2 years follow-up.
Efficacy was also assessed in patients who were intolerant to imatinib. In this population of patients who received 100 mg once daily, MCyR was achieved in 77% and CCyR in 67% with a minimum of 2 years follow-up.
Approximately 20% of subjects remained on study therapy through study completion (i.e. a minimum of 7 years), discontinuing study treatment due to study closure. Table 12 represents the comparative data (1 - 7 years), for the recommended starting dose 100 mg once daily in CML chronic phase.
 In the Phase III, randomized, open-label study in patients with advanced phase CML and Ph+ALL, whose disease was resistant to or who were intolerant to imatinib, the primary endpoint was MaHR. A total of 611 patients were randomised to either the dasatinib 140 mg once daily or 70 mg twice daily group. Median duration of treatment was approximately 6 months (range < 1-31 months).
In the Phase III, randomized, open-label study in patients with advanced phase CML and Ph+ALL, whose disease was resistant to or who were intolerant to imatinib, the primary endpoint was MaHR. A total of 611 patients were randomised to either the dasatinib 140 mg once daily or 70 mg twice daily group. Median duration of treatment was approximately 6 months (range < 1-31 months).
The once daily schedule demonstrated comparable efficacy (non-inferiority) to the twice daily schedule on the primary efficacy endpoint (difference in MaHR 0.8%; 95% confidence interval [-7.1% - 8.7%]); The response rates are presented in Table 13.
 In patients with accelerated phase CML treated with the 140 mg once daily regimen, the median duration of MaHR and the median overall survival in patients with accelerated phase CML was not reached for either group; the median PFS was 25 months and 26 months for the 140 mg once daily group and the 70 mg twice daily group, respectively; and the median overall survival was not reached for the 140 mg once daily group and 31 months for the 70 mg twice daily group.
In patients with accelerated phase CML treated with the 140 mg once daily regimen, the median duration of MaHR and the median overall survival in patients with accelerated phase CML was not reached for either group; the median PFS was 25 months and 26 months for the 140 mg once daily group and the 70 mg twice daily group, respectively; and the median overall survival was not reached for the 140 mg once daily group and 31 months for the 70 mg twice daily group.
In patients with myeloid blast phase CML treated with the 140 mg once daily regimen the median duration of MaHR was 8 months, and 9 months for the 140 mg once daily group and the 70 mg twice daily group, respectively; the median PFS was 4 months for both groups; and the median overall survival was 8 months for both groups. In patients with lymphoid blast phase CML, the median duration of MaHR was 5 months and 8 months for the 140 mg once daily group and the 70 mg twice daily group, respectively; the median PFS was 5 months for both groups, and the median overall survival was 11 months and 9 months, respectively.
In patients with Ph+ ALL treated with the 140 mg once daily regimen, the median duration of MaHR was 5 months and 12 months for the 140 mg once daily group and the 70 mg twice daily group, respectively; the median PFS was 4 months and 3 months respectively, and the median overall survival was 7 months and 9 months, respectively.
Ph+ ALL - newly diagnosed adults.
A total of 426 newly diagnosed adult Ph+ ALL patients have been treated with dasatinib integrated with chemotherapy in 11 phase II clinical trials. The efficacy results from 4 pivotal phase II studies are summarised below.
In a phase II study, Ravandi et al reported on seventy-two (72) newly diagnosed Ph+ ALL patients (median age 55, range 21 - 80 years) treated with dasatinib (100 mg once a day or 50 mg twice a day on days 1-14 of each chemotherapy cycle) integrated with a HyperCVAD chemotherapy protocol during induction, followed by dasatinib 100 mg once a day or 50 mg twice a day as maintenance. Sixty nine (96%) achieved CR. Among them, 57 (83%) achieved cytogenetic CR after 1 cycle, and 64 (93%) achieved a major molecular response at a median of 4 weeks (range, 2-38 weeks). Sixty-five patients (94%) were negative for minimal residual disease assessed by flow cytometry at a median of 3 weeks (range, 2-37 weeks). With a median follow-up of 67 months (range, 33-97 months), 33 patients (46%) were alive, and 30 (43%) were in CR. The 5 year disease free survival (DFS) and overall survival (OS) outcomes estimated via Kaplan-Meier method were 44% and 46% respectively.
Yoon et al reported on a phase II clinical study in 51 newly diagnosed Ph+ ALL patients (median age 46 range 19-64 years) treated with 2 cycles of dasatinib (100 mg once daily) integrated with chemotherapy (each cycle included 4 weeks) during induction and upto 4 cycles as consolidation followed by 2 years of maintenance with dasatinib. After the first dasatinib cycle, 50 patients (98.0%) achieved CR. By the end of the second dasatinib cycle, 46 (93.9%) of 49 assessable patients had persistent CR with 39 progressing to allogenic stem cell transplant. After a median follow-up of 54 months (range 40-63), the 4-year cumulative incidence of DFS and OS rate for all patients were 52.0% and 51%, respectively.
Rousellot et al investigated dasatinib, in combination with low-intensity chemotherapy in 71 newly diagnosed patients with Ph+ All, over 55 years of age (median 69, range 59 - 83). Patients were treated with dasatinib 140 mg/day (100 mg/day over 70 years) plus intrathecal chemotherapy, vincristine, and dexamethasone during induction. Patients in complete remission continued consolidation with dasatinib, sequentially with cytarabine, asparaginase, and methotrexate for 6 months. Maintenance therapy was dasatinib and vincristine/dexamethasone reinductions for 18 months followed by dasatinib until relapse or death. 96% of patients achieved CR at induction and 7 patients underwent allogenic stem cell transplant. After a median follow up of 66 months (21 - 88) 5 year Event Free Survival (EFS), and OS were 27% (95% CI, 17-37), and 36% (95% CI, 25-47) respectively.
In the GIMEMA LAL1205 study published by Foa et al, 53 patients with newly diagnosed Ph+ ALL older than 18 years (median age 54, range 23-76 years) received dasatinib (70 mg twice daily) induction therapy for 84 days combined with steroids for the first 32 days and intrathecal chemotherapy. Post remission therapy was not specified. All patients achieved a complete hematologic remission (CHR). At 20 months, the overall survival was 69.2% and disease-free survival was 51.1%.
Chronic phase CML - paediatric patients.
The efficacy of dasatinib in paediatric patients was evaluated in two paediatric studies in which 97 patients with chronic phase CML received dasatinib tablets. Among 97 patients with chronic phase CML treated in two paediatric studies, an open-label, non-randomized dose-ranging trial (NCT00306202) and an open-label, non-randomized, single-arm trial (NCT00777036), 51 patients (exclusively from the single-arm trial) had newly diagnosed chronic phase CML and 46 patients (17 from the dose-ranging trial; 29 from the single-arm trial) were resistant or intolerant to previous treatment with imatinib. Ninety-one of the 97 paediatric patients were treated with dasatinib tablets 60 mg/m2 once daily (maximum dose of 100 mg once daily for patients with high BSA). Patients were treated until disease progression or unacceptable toxicity.
Baseline demographic characteristics of the 46 imatinib resistant or intolerant patients were: median age 13.5 years (range 2 to 20 years), 78.3% White, 15.2% Asian, 4.4% Black, 2.2% other, and 52% female. Baseline characteristics of the 51 newly diagnosed patients were: median age 12.8 years (range 1.9 to 17.8 years), 60.8% White, 31.4% Asian, 5.9% Black, 2% Other, and 49% female.
Median duration of follow-up was 5.2 years (range 0.5 to 9.3 years) for the imatinib resistant or intolerant patients and 4.5 years (range 1.3 to 6.4 years) for the newly diagnosed patients, respectively. Efficacy results for the two paediatric studies are summarised in Table 14.
Table 14 shows increasing trend for response for CCyR, MCyR, and MMR across time (3 months to 24 months). The increasing trend in response for all three endpoints is seen in both the newly diagnosed and imatinib resistant or intolerant patients.
 With a median follow-up of 4.5 years in newly diagnosed patients, the median durations of CCyR, MCyR, MMR could not be estimated as more than half of the responding patients had not progressed at the time of data cut-off. Range of duration of response was (2.5+ to 66.5+ months for CCyR), (1.4 to 66.5+ months for MCyR), and (5.4+ to 72.5+ months for subjects who achieved MMR by month 24 and 0.03+ to 72.5+ months for subjects who achieved MMR at any time), where '+' indicates a censored observation.
With a median follow-up of 4.5 years in newly diagnosed patients, the median durations of CCyR, MCyR, MMR could not be estimated as more than half of the responding patients had not progressed at the time of data cut-off. Range of duration of response was (2.5+ to 66.5+ months for CCyR), (1.4 to 66.5+ months for MCyR), and (5.4+ to 72.5+ months for subjects who achieved MMR by month 24 and 0.03+ to 72.5+ months for subjects who achieved MMR at any time), where '+' indicates a censored observation.
With a median follow-up of 5.2 years in imatinib-resistant or - intolerant patients, the median durations of CCyR, MCyR, and MMR could not be estimated as more than half the responding patients had not progressed at the time of data cut-off. Range of duration of response was (2.4 to 86.9+ months for CCyR), (2.4 to 86.9+ months for MCyR), and (2.6+ to 73.6+ months for MMR), where '+' indicates a censored observation.
The median time to response for MCyR was 2.9 months (95% CI: 2.8 months, 3.5 months) in the pooled imatinib-resistant/intolerant CP-CML patients. The median time to response for CCyR was 3.3 months (95% CI: 2.8 months, 4.7 months) in the pooled imatinib-resistant/intolerant CP-CML patients. The median time to response for MMR was 8.3 months (95% CI: 5.0 months, 11.8 months) in the pooled imatinib-resistant/intolerant CP-CML patients.
The median time to response for MCyR was 3.0 months (95% CI: 2.8 months, 4.3 months) in the newly diagnosed treatment-naïve CP-CML patients. The median time to response for CCyR was 5.5 months (95% CI: 3.0 months, 5.7 months) in the newly diagnosed treatment-naïve CP-CML patients. The median time to response for MMR was 8.9 months (95% CI: 6.2 months, 11.7 months) in the newly diagnosed treatment-naïve CP-CML patients.
In the Phase II pediatric study, 1 newly diagnosed patient and 2 imatinib-resistant or intolerant patients progressed to blast phase CML.
Ph+ ALL - paediatric patients.
The efficacy of dasatinib in combination with chemotherapy was evaluated in a single cohort of Study CA180372 (NCT01460160), a multicenter study of paediatric patients with newly diagnosed B-cell precursor Ph+ ALL. Eighty-two patients received dasatinib tablets at a daily dose of 60 mg/m2 for up to 24 months, in combination with chemotherapy. The backbone chemotherapy regimen was the AIEOP-BFM ALL 2000 multi-agent chemotherapy protocol.
Patients had a median age of 10.4 years (range 2.6 to 17.9 years) and included 21 patients (25.6%) 2 to 6 years of age, 27 patients (32.9%) 7 to 12 years of age, and 34 patients (41.5%) 13 to 17 years of age. Eighty percent of patients were white, and 55% were male. Thirty-two patients (41%) had a white blood cell count (WBC) of ≥ 50,000/microL at diagnosis, and 17 patients (20.7%) had extramedullary disease.
Efficacy was established on the basis of 3-year event-free survival (EFS), defined as the time from the start of dasatinib to lack of complete response at the end of the third high risk block, relapse, secondary malignancy, or death from any cause. The 3-year EFS rate for patients on Study CA180372 was 65.1% (95% CI: 53.6, 74.4). At the end of induction, 72 patients (87.8%) had a bone marrow with < 5% lymphoblasts, and 77 patients (93.9%) achieved this by the end of consolidation.
The minimal residual disease (MRD) negativity rate assessed by Ig/TCR rearrangement was 74.4% by the end of consolidation in all treated patients. When this rate was based on the 70 patients with evaluable Ig/TCR assessments, the estimate was 87.1%.
5.2 Pharmacokinetic Properties
The pharmacokinetics of dasatinib were evaluated in 235 healthy subjects and in 84 patients with leukaemia.
Absorption.
Dasatinib is rapidly absorbed in patients following oral administration. The absolute bioavailability of dasatinib has not been determined. Peak concentrations were observed between 0.5-6 hours. Following oral administration, the increase in the mean exposure (AUCT) is approximately proportional to the dose increment across doses ranging from 15 mg to 240 mg daily.
Data from a study of healthy subjects administered a single, 100 mg dose of dasatinib 30 minutes following consumption of a high-fat meal indicated a 14% increase in the mean AUC of dasatinib. Consumption of a low-fat meal 30 minutes prior to dasatinib resulted in a 21% increase in the mean AUC of dasatinib. The observed food effects are unlikely to be clinically significant. Dasatinib exposure variability is higher under fasted conditions (47% CV) compared to light-fat meal (39% CV) and high-fat meal (32% CV) conditions.
Based on the patient population PK analysis, variability in dasatinib exposure was estimated to be mainly due to inter-occasion variability in bioavailability (44% CV) and, to a lesser extent, due to inter-individual variability in bioavailability and inter-individual variability in clearance (30% and 32% CV, respectively). The random inter-occasion variability in exposure is not expected to affect the cumulative exposure and efficacy.
Distribution.
In patients, dasatinib has a large apparent volume of distribution (2505 L) suggesting that the drug is extensively distributed in the extravascular space.
Metabolism.
Dasatinib is extensively metabolized in humans. In a study of 8 healthy subjects administered 100 mg of [14C]-labelled dasatinib, unchanged dasatinib represented 29% of circulating radioactivity in plasma. Plasma concentration and measured in vitro activity indicate that metabolites of dasatinib are unlikely to play a major role in the observed pharmacology of the drug. The overall mean terminal half-life of dasatinib is approximately 5-6 hours. CYP3A4 is a major enzyme responsible for the metabolism of dasatinib.
Excretion.
Elimination is predominantly in the faeces, mostly as metabolites. Following a single oral dose of [14C]-labelled dasatinib, approximately 89% of the dose was eliminated within 10 days, with 4% and 85% of the administered radioactivity recovered in the urine and faeces, respectively. Unchanged dasatinib accounted for 0.1% and 19% of the administered dose in urine and faeces, respectively, with the remainder of the dose being metabolites.
Special populations.
Age and gender.
Pharmacokinetic analyses of demographic data indicate that there are no clinically relevant effects of age and gender on the pharmacokinetics of dasatinib.
Hepatic impairment.
The effect of hepatic impairment on the single-dose pharmacokinetics of dasatinib was assessed in 8 moderately hepatic-impaired subjects who received a 50 mg dose and 5 severely hepatic-impaired subjects who received a 20 mg dose compared to matched healthy subjects who received a 70 mg dose of dasatinib. The mean Cmax and AUC of dasatinib adjusted for the 70 mg dose was decreased by 47% and 8%, respectively, in subjects with moderate hepatic impairment compared to subjects with normal hepatic function. In severely hepatic-impaired subjects, the mean Cmax and AUC adjusted for the 70 mg dose was decreased by 43% and 28% respectively, compared to subjects with normal hepatic function (see Section 4.4 Special Warnings and Precautions for Use, Use in hepatic impairment; Section 4.2 Dose and Method of Administration, Special populations).
Renal impairment.
There are no clinical studies of dasatinib in patients with impaired renal function. Less than 4% of dasatinib and its metabolites are excreted via the kidney (see Section 4.4 Special Warnings and Precautions for Use, Use in renal impairment; Section 4.2 Dose and Method of Administration, Special populations).
Paediatric patients.
The pharmacokinetics of dasatinib film-coated tablets was evaluated in 72 paediatric patients with relapsed or refractory leukaemia or solid tumors at oral doses ranging from 60 to 120 mg/m2 once daily and 50 to 110 mg/m2 twice daily. Data was pooled across two studies and showed that the tablet was rapidly absorbed. Mean Tmax was observed between 0.5 and 6 hours and mean half-life ranged from 2 to 5 hours across all dose levels and age groups. Dasatinib PK showed dose proportionality with a dose related increase in exposure observed in paediatric patients. There was no significant difference of dasatinib PK between children and adolescents. The geometric means of dose-normalized dasatinib Cmax, AUC 0-T, and AUC(INF) appeared to be similar between children and adolescents at different dose levels. A population pharmacokinetic (PPK) model-based simulation predicted that the body weight tiered dosing recommendation described for dasatinib tablets (see Section 4.2 Dose and Method of Administration, Paediatric dosage) is expected to provide similar exposure to a tablet dose of 60 mg/m2. The bioavailability of dispersed tablets in paediatric patients was estimated to be 36% lower than that of intact tablets.
5.3 Preclinical Safety Data
Genotoxicity.
Dasatinib was not mutagenic when tested in in vitro bacterial cell assays (Ames test) and was not clastogenic in an in vivo rat micronucleus study. Clastogenicity was observed with dasatinib in vitro in assays with Chinese hamster ovary cells in the absence and presence of metabolic activation.
Carcinogenicity.
In a two year carcinogenicity study, rats were administered oral doses of dasatinib at 0.3, 1, and 3 mg/kg/day. The highest dose resulted in a plasma drug exposure (AUC) level generally equivalent to or slightly lower than calculated human exposure at the recommended range of starting doses 100 mg or 140 mg daily. A statistically significant increase in the combined incidence of squamous cell carcinomas and papillomas in the uterus and cervix of high-dose female rats and of prostate adenoma in low-dose male rats was noted.6 Pharmaceutical Particulars
6.1 List of Excipients
Tablet core.
Lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, hyprolose, magnesium stearate.
Film coating.
Titanium dioxide, hypromellose, triethyl citrate.
6.2 Incompatibilities
Incompatibilities were either not assessed or not identified as part of the registration of this medicine.
6.3 Shelf Life
In Australia, information on the shelf life can be found on the public summary of the Australian Register of Therapeutic Goods (ARTG). The expiry date can be found on the packaging.
6.4 Special Precautions for Storage
Dasatinib Sandoz tablets should be stored below 25°C. Store in original container.
6.5 Nature and Contents of Container
Dasatinib Sandoz is available in 60 cc white round opaque HDPE bottles with 1 g silica desiccant canister (labelled 'Do Not Eat') and PP CRC (white) with cotton wadding (Puritan cotton coil) and an induction seal.
The 20 mg, 50 mg and 70 mg tablets are available in bottles of 60 tablets. The 100 mg tablets are available in bottles of 30 tablets.
Not all pack sizes may be marketed.
6.6 Special Precautions for Disposal
Procedures for proper handling and disposal of anticancer drugs should be considered. Several guidelines on this subject have been published. There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate.
Dasatinib Sandoz tablets consist of a core tablet (containing the active drug substance), surrounded by a film coating to prevent exposure of pharmacy and clinical personnel to the active drug substance. However, if tablets are crushed or broken, pharmacy and clinical personnel should wear disposable chemotherapy gloves. Personnel who are pregnant should avoid exposure to crushed and/or broken tablets.
6.7 Physicochemical Properties
Chemical structure.
Dasatinib drug substance has the following chemical structure:
 The chemical name for dasatinib is N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl]-2-methyl-4-pyrimidinyl]amino]-5-thiazolecarboxamide.
The chemical name for dasatinib is N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl]-2-methyl-4-pyrimidinyl]amino]-5-thiazolecarboxamide.
The molecular formula is C22H26ClN7O2S, which corresponds to a formula weight of 488.01.
The drug substance is insoluble in water (0.008 mg/mL) at 24 ± 4°C. The pH of a saturated solution of dasatinib in water is about 6.0. Two basic ionization constants (pKa) were determined to be 6.8 and 3.1, and one weakly acidic pKa was determined to be 10.8. The solubilities of dasatinib in various solvents at 24 ± 4°C are as follows: slightly soluble in ethanol (USP), methanol, polyethylene glycol 400, and propylene glycol; very slightly soluble in acetone and acetonitrile; and practically insoluble in corn oil.
CAS number.
The CAS number for dasatinib monohydrate is 863127-77-9.7 Medicine Schedule (Poisons Standard)
S4 - Prescription Only Medicine.
Summary Table of Changes




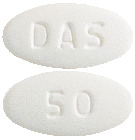
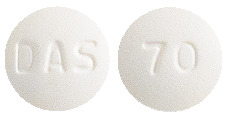

 Dose escalation is not recommended for paediatric patients with Ph+ ALL, as dasatinib is administered in combination with chemotherapy in these patients.
Dose escalation is not recommended for paediatric patients with Ph+ ALL, as dasatinib is administered in combination with chemotherapy in these patients.
 For paediatric patients with chronic phase CML, if Grade ≥ 3 neutropenia or thrombocytopenia recurs during complete haematologic response (CHR), Dasatinib Sandoz should be interrupted, and may be subsequently resumed at a reduced dose. Temporary dose reductions for intermediate degrees of cytopenia and disease response should be implemented as needed.
For paediatric patients with chronic phase CML, if Grade ≥ 3 neutropenia or thrombocytopenia recurs during complete haematologic response (CHR), Dasatinib Sandoz should be interrupted, and may be subsequently resumed at a reduced dose. Temporary dose reductions for intermediate degrees of cytopenia and disease response should be implemented as needed.
 Myelosuppression was commonly reported in all patient populations. In newly diagnosed chronic phase CML, myelosupression was less frequently reported than in chronic phase CML patients with resistance or intolerance to prior imatinib therapy. The frequency of Grade 3 or 4 neutropenia, thrombocytopenia, and anaemia was higher in patients with advanced CML or Ph+ ALL than in chronic phase CML.
Myelosuppression was commonly reported in all patient populations. In newly diagnosed chronic phase CML, myelosupression was less frequently reported than in chronic phase CML patients with resistance or intolerance to prior imatinib therapy. The frequency of Grade 3 or 4 neutropenia, thrombocytopenia, and anaemia was higher in patients with advanced CML or Ph+ ALL than in chronic phase CML.
 For time-to cCCyR, a hazard ratio of 1.55 indicates that a patient treated with dasatinib is 55% more likely to achieve a cCCyR at any time compared to a patient treated with imatinib. Similarly, for time-to MMR, a hazard ratio of 2.01 indicates a patient treated with dasatinib is more than two times more likely to achieve a MMR at any time compared to a patient treated with imatinib. For durability of cCCyR (time-in response), a hazard ratio of 0.7 indicates a patient treated with dasatinib is 30% less likely to have disease progression after achieving a cCCyR (or never achieving a cCCyR) compared to a patient treated with imatinib.
For time-to cCCyR, a hazard ratio of 1.55 indicates that a patient treated with dasatinib is 55% more likely to achieve a cCCyR at any time compared to a patient treated with imatinib. Similarly, for time-to MMR, a hazard ratio of 2.01 indicates a patient treated with dasatinib is more than two times more likely to achieve a MMR at any time compared to a patient treated with imatinib. For durability of cCCyR (time-in response), a hazard ratio of 0.7 indicates a patient treated with dasatinib is 30% less likely to have disease progression after achieving a cCCyR (or never achieving a cCCyR) compared to a patient treated with imatinib.
 The progression-free survival rate by specific timepoint is displayed graphically in Figure 1. Rate of PFS was consistently higher in dasatinib-treated patients who achieved BCR-ABL level ≤ 10 percent at 3 months than those who did not.
The progression-free survival rate by specific timepoint is displayed graphically in Figure 1. Rate of PFS was consistently higher in dasatinib-treated patients who achieved BCR-ABL level ≤ 10 percent at 3 months than those who did not. The overall survival rate by specific timepoint is displayed graphically in Figure 2. Rate of OS was consistently higher in dasatinib-treated patients who achieved BCR-ABL level ≤ 10 percent at 3 months than those who did not.
The overall survival rate by specific timepoint is displayed graphically in Figure 2. Rate of OS was consistently higher in dasatinib-treated patients who achieved BCR-ABL level ≤ 10 percent at 3 months than those who did not. The time to MMR is displayed graphically in Figure 3. The time to MMR was consistently shorter in dasatinib-treated subjects compared with imatinib-treated subjects.
The time to MMR is displayed graphically in Figure 3. The time to MMR was consistently shorter in dasatinib-treated subjects compared with imatinib-treated subjects. MMR rates by specific timepoint are displayed graphically in Figure 4. Rates of MMR were consistently higher in dasatinib-treated subjects compared with imatinib-treated subjects.
MMR rates by specific timepoint are displayed graphically in Figure 4. Rates of MMR were consistently higher in dasatinib-treated subjects compared with imatinib-treated subjects. MR4.5 rates over time are displayed graphically in Figure 5. Rate of MR4.5 over time was consistently higher in dasatinib-treated subjects compared with imatinib-treated subjects.
MR4.5 rates over time are displayed graphically in Figure 5. Rate of MR4.5 over time was consistently higher in dasatinib-treated subjects compared with imatinib-treated subjects. Disease progression was defined as increasing white blood cells despite appropriate therapeutic management, loss of CHR (complete haematologic response), partial CyR or CCyR, progression to accelerated phase or blast phase, or death. The estimated 60-month PFS rate was 88.9% (CI: 84.0% - 92.4%) and 89.2% (CI: 84.3% - 92.7%) for the dasatinib and imatinib treatment groups, respectively. Transformation to accelerated or blast phase occurred less frequently with dasatinib (n = 8; 3.1%) than with imatinib-treated patients (n = 15; 5.8%). The estimated 60-month survival rates for dasatinib and imatinib-treated patients were 90.9% (CI: 86.6% - 93.8%) and 89.6% (CI: 85.2% - 92.8%) respectively.
Disease progression was defined as increasing white blood cells despite appropriate therapeutic management, loss of CHR (complete haematologic response), partial CyR or CCyR, progression to accelerated phase or blast phase, or death. The estimated 60-month PFS rate was 88.9% (CI: 84.0% - 92.4%) and 89.2% (CI: 84.3% - 92.7%) for the dasatinib and imatinib treatment groups, respectively. Transformation to accelerated or blast phase occurred less frequently with dasatinib (n = 8; 3.1%) than with imatinib-treated patients (n = 15; 5.8%). The estimated 60-month survival rates for dasatinib and imatinib-treated patients were 90.9% (CI: 86.6% - 93.8%) and 89.6% (CI: 85.2% - 92.8%) respectively. Efficacy was also assessed in patients who were intolerant to imatinib. In this population of patients who received 100 mg once daily, MCyR was achieved in 77% and CCyR in 67% with a minimum of 2 years follow-up.
Efficacy was also assessed in patients who were intolerant to imatinib. In this population of patients who received 100 mg once daily, MCyR was achieved in 77% and CCyR in 67% with a minimum of 2 years follow-up. In the Phase III, randomized, open-label study in patients with advanced phase CML and Ph+ALL, whose disease was resistant to or who were intolerant to imatinib, the primary endpoint was MaHR. A total of 611 patients were randomised to either the dasatinib 140 mg once daily or 70 mg twice daily group. Median duration of treatment was approximately 6 months (range < 1-31 months).
In the Phase III, randomized, open-label study in patients with advanced phase CML and Ph+ALL, whose disease was resistant to or who were intolerant to imatinib, the primary endpoint was MaHR. A total of 611 patients were randomised to either the dasatinib 140 mg once daily or 70 mg twice daily group. Median duration of treatment was approximately 6 months (range < 1-31 months). In patients with accelerated phase CML treated with the 140 mg once daily regimen, the median duration of MaHR and the median overall survival in patients with accelerated phase CML was not reached for either group; the median PFS was 25 months and 26 months for the 140 mg once daily group and the 70 mg twice daily group, respectively; and the median overall survival was not reached for the 140 mg once daily group and 31 months for the 70 mg twice daily group.
In patients with accelerated phase CML treated with the 140 mg once daily regimen, the median duration of MaHR and the median overall survival in patients with accelerated phase CML was not reached for either group; the median PFS was 25 months and 26 months for the 140 mg once daily group and the 70 mg twice daily group, respectively; and the median overall survival was not reached for the 140 mg once daily group and 31 months for the 70 mg twice daily group. With a median follow-up of 4.5 years in newly diagnosed patients, the median durations of CCyR, MCyR, MMR could not be estimated as more than half of the responding patients had not progressed at the time of data cut-off. Range of duration of response was (2.5+ to 66.5+ months for CCyR), (1.4 to 66.5+ months for MCyR), and (5.4+ to 72.5+ months for subjects who achieved MMR by month 24 and 0.03+ to 72.5+ months for subjects who achieved MMR at any time), where '+' indicates a censored observation.
With a median follow-up of 4.5 years in newly diagnosed patients, the median durations of CCyR, MCyR, MMR could not be estimated as more than half of the responding patients had not progressed at the time of data cut-off. Range of duration of response was (2.5+ to 66.5+ months for CCyR), (1.4 to 66.5+ months for MCyR), and (5.4+ to 72.5+ months for subjects who achieved MMR by month 24 and 0.03+ to 72.5+ months for subjects who achieved MMR at any time), where '+' indicates a censored observation. The chemical name for dasatinib is N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl]-2-methyl-4-pyrimidinyl]amino]-5-thiazolecarboxamide.
The chemical name for dasatinib is N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl]-2-methyl-4-pyrimidinyl]amino]-5-thiazolecarboxamide.