SUMMARY CMI
Diltiazem Sandoz CD Capsules®
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
1. Why am I using Diltiazem Sandoz CD Capsules?
Diltiazem Sandoz CD contains an active ingredient diltiazem hydrochloride. Diltiazem Sandoz CD Capsules are used to prevent angina or to treat hypertension (high blood pressure).
For more information, see Section 1. Why am I using Diltiazem Sandoz CD Capsules? in the full CMI.
2. What should I know before I use Diltiazem Sandoz CD Capsules?
Do not use if you have ever had an allergic reaction to Diltiazem Sandoz CD Capsules or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I use Diltiazem Sandoz CD Capsules? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with Diltiazem Sandoz CD Capsules and affect how it works.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I use Diltiazem Sandoz CD Capsules?
- Diltiazem Sandoz CD Capsules can be taken once a day, preferably at the same time every day.
- Swallow the capsules or the tablets with a glass of water. Do not chew them.
- Your doctor will tell you how often and how much Diltiazem Sandoz CD to take.
More instructions can be found in Section 4. How do I use Diltiazem Sandoz CD Capsules? in the full CMI.
5. What should I know while using Diltiazem Sandoz CD Capsules?
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using Diltiazem Sandoz CD Capsules? in the full CMI.
6. Are there any side effects?
All medicines can have side effects. Sometimes they are serious, but most of the time they are not. Tell your doctor or pharmacist as soon as possible if you do not feel well while you are using these medicines. Some of the serious side effects are heat beating irregularly, slowly or very quickly; continuously lightheaded or dizzy; feeling pain, which may be severe, in your left arm and chest. For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
FULL CMI
Diltiazem Sandoz CD Capsules®
Active ingredient: Diltiazem hydrochloride
Consumer Medicine Information (CMI)
This leaflet provides important information about using Diltiazem Sandoz CD Capsules. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using Diltiazem Sandoz CD Capsules.
Where to find information in this leaflet:
1. Why am I using Diltiazem Sandoz CD Capsules?
2. What should I know before I use Diltiazem Sandoz CD Capsules?
3. What if I am taking other medicines?
4. How do I use Diltiazem Sandoz CD Capsules?
5. What should I know while using Diltiazem Sandoz CD Capsules?
6. Are there any side effects?
7. Product details
1. Why am I using Diltiazem Sandoz CD Capsules?
Diltiazem Sandoz CD Capsules contains an active ingredient called diltiazem hydrochloride. Diltiazem Sandoz CD Capsules is designed to release the active ingredient slowly so that it works over 24 hours and can be taken once a day (CD stands for "controlled delivery").
This medicine belongs to a group of medicines called calcium channel blockers or calcium antagonists. They work by opening up blood vessels, which lowers blood pressure and lets more blood and oxygen reach the heart. They do not change the amount of calcium in your blood or bones.
Diltiazem Sandoz CD Capsules is used to prevent angina or to treat hypertension (high blood pressure).
Angina is a pain or uncomfortable sensation in the chest, often spreading to the arms or neck and sometimes to the shoulders and back. The pain of angina is due to a shortage of oxygen to the heart.
High blood pressure can have many different causes, including kidney disease, hardening of the arteries and some hormone imbalances. However, the vast majority of people with high blood pressure have no identifiable cause for it. If left untreated, high blood pressure can lead to serious health problems such as a stroke or heart attack.
Your doctor may have prescribed these medicines for another reason.
Ask your doctor if you have any questions about why these medicines have been prescribed for you.
There is no evidence that these medicines are addictive.
These medicines are available only with a doctor's prescription.
2. What should I know before I use Diltiazem Sandoz CD Capsules?
Warnings
Do not use Diltiazem Sandoz CD Capsules if:
- you are allergic to diltiazem hydrochloride, or any of the ingredients listed at the end of this leaflet.
Symptoms of an allergic reaction to these medicines may include:
- asthma, wheezing or shortness of breath
- swelling of the face, lips or tongue, which may cause difficulty in swallowing or breathing
- hives, itching or skin rash
- fainting
Always check the ingredients to make sure you can use this medicine. - you have certain types of abnormal heart rhythm
- you have hypotension (low blood pressure)
- you have had a heart attack or have other heart-related complications
- you have pulmonary congestion (fluid on the lungs)
- you are currently taking any of the following medications:
- dantrolene (muscle relaxant)
- ivabradine (for heart failure/angina) - the packaging is torn or shows signs of tampering or if tablets or capsules do not look right
- the expiry date (EXP) printed on the pack has passed
If you take these medicines after the expiry date has passed, it may not work as well.
Check with your doctor if you:
- dabigatran, rivaroxaban, apixaban (blood thinners such as direct acting oral anti-coagulants)
- are not sure whether you should start taking these medicines
- take any medicines for any other condition
- have allergies to:
- any other medicines
- any other substances, such as foods, preservatives or dyes - have or have had any medical conditions, especially the following:
- abnormal heart beat rhythm
- hypotension (low blood pressure)
- heart attack or other heart-related complications
- impaired renal (kidney) or hepatic (liver) function
- diabetes
- asthma
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Use in Children
Do not give these medicines to a child.
The safety and effectiveness of these medicines have not been established in children.
Pregnancy and breastfeeding
Talk to your doctor if you are pregnant or intend to become pregnant.
Diltiazem Sandoz CD capsules should not be used during pregnancy.
These medicines may affect your developing baby if they are taken during pregnancy.
Talk to your doctor if you are breastfeeding or intend to breastfeed.
Your doctor will discuss this situation with you. A decision will have to be made whether to discontinue breastfeeding or discontinue therapy taking into consideration the importance of the medicine.
The active ingredient of these medicines passes into breast milk and may affect your baby.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interfere with Diltiazem Sandoz CD Capsules and affect how it works. These include:
- dantrolene (a muscle relaxant)
- aspirin
- medications used to help prevent blood clots (antiplatelets)
- cilostazol (a medicine used to treat blockage of blood vessels to the legs)
- Some other medicines for your heart or high blood pressure (eg. beta blockers, digoxin, amiodarone, nitrates)
- ciclosporin, which you may have been given after an operation or because of rheumatoid arthritis
- rifampicin (an antibiotic)
- cimetidine or ranitidine (for ulcers or reflux)
- diazepam (for depression, alcohol withdrawal or anxiety)
- phenytoin (for epilepsy)
- carbamazepine (for bipolar disorder or epilepsy)
- lithium (for bipolar disorder)
- theophylline (for asthma and other breathing problems)
- certain drugs used to treat prostate problems
- ivabradine (for heart failure/angina)
- inhaled anaesthetic agents such as halothane, isoflurane, enflurane (for surgery)
- drugs used to lower your blood cholesterol (including simvastatin, lovastatin)
- benzodiazepines or medicines used as sedatives or to treat anxiety such as midazolam, triazolam
- corticosteroids such as methylprednisolone, prednisone, cortisone
- antiarrhythmics or medicines used to treat irregular heart beats
- medicines used during scans to see images of your body
- colchicine (drug used to treat gout) when administered concomitantly
- blood thinners such as direct acting oral anticoagulants such as dabigatran, rivaroxaban, apixaban
- drugs with the potential/known for prolonging the QT interval.
Diltiazem Sandoz CD capsules may themselves be affected, or they may affect how well these medicines work. You may need to take different amounts of your medicine, or you may need to take different medicines. Your doctor will advise you.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect Diltiazem Sandoz CD Capsules.
4. How do I use Diltiazem Sandoz CD Capsules?
How much to take
- Diltiazem Sandoz CD capsules can be taken once a day, preferably at the same time every day.
Your doctor will tell you how often and how many Diltiazem Sandoz CD capsules to take.
Follow all directions given to you by your doctor and pharmacist carefully until your doctor tells you to stop. Write them down if necessary.
If you do not understand the instructions on the packaging of these medicines, ask your doctor or pharmacist for help.
How to take it
Swallow the capsules or the tablets with a glass of water. Do not chew them.
When to take it
Take these medicines at the same time(s) every day.
If you forget to use Diltiazem Sandoz CD Capsules
If you are taking these medicines for angina, do not suddenly stop taking them since this can cause severe angina for a day or two.
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to. Otherwise, take it as soon as you remember, and then go back to taking your tablets or capsules as you would normally.
Do not take a double dose to make up for the dose you missed.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you use too much Diltiazem Sandoz CD Capsules
If you think that you have used too much Diltiazem Sandoz CD Capsules, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
(by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
If you take too much of these medicines, you may:
- feel continuously light-headed or dizzy
- notice your heart beating very slowly
- feel pain, which could be severe, in your left arm and chest.
- Excess fluid may accumulate in your lungs (pulmonary oedema) causing shortness of breath that may develop up to 24-48 hours after intake.
If any of these occur, you should get medical attention immediately.
5. What should I know while using Diltiazem Sandoz CD Capsules?
Things you should do
Take these medicines exactly as your doctor has prescribed.
If you do not follow your doctor's instructions, you may not get relief from your attacks of angina, or your blood pressure may not be as well controlled as it could be.
If you are taking these medicines for angina, tell your doctor if you continue to have angina attacks or if they become more frequent.
Tell your doctor dentist or pharmacist that you are taking Diltiazem Sandoz CD capsules if you are about to be started on any new medicine.
Things to be careful of
Be careful not to overdo physical activities when you first start using these medicines.
You may feel better when you start taking these medicines, but you will need time to improve your physical fitness.
Drinking grapefruit juice may increase the effects of Diltiazem Sandoz CD capsules.
Get up slowly when getting out of bed or standing up if you feel light-headed, dizzy or faint.
If this is a problem and it gets worse or continues, talk to your doctor.
Things you should not do
- Do not use these medicines to treat any other complaints unless your doctor says to.
- Do not give these medicines to anyone else, even if they have the same condition as you.
- As mentioned previously, if you are taking these medicines for angina, do not suddenly stop taking your medicine since this can cause severe angina for a day or two.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how these medicines affect you.
These medicines may cause dizziness and fainting in some patients, especially when you first start to use them. Make sure you know how you react to these medicines before you drive a car, operate machinery, or do anything else that could be dangerous if this happens to you.
Looking after your medicine
- Keep these medicines in their packaging until it is time to take them. If you take the medicine out of its container it may not keep well.
- Store below 25°C.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on window sills.
A locked cupboard at least one-and-a half metres above the ground is a good place to store medicines.
Keep it where young children cannot reach it.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
These medicines help most people with angina, and Diltiazem Sandoz CD capsules will help control most people's blood pressure, but they may have unwanted effects in a few people.
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
Skin-related
| Speak to your doctor if you have any of these less serious side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
General
| Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects as follows:
Australia: Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems
By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What Diltiazem Sandoz CD Capsules contains
Diltiazem Sandoz CD 180 and 240 mg capsules
| Active ingredient (main ingredient) | Diltiazem hydrochloride 180 mg or 240 mg |
| Other ingredients (inactive ingredients) | fumaric acid, purified talc, non-pareil seeds (sucrose), colloidal anhydrous silica, white beeswax, ethylcellulose, castor oil, stearic acid, methacrylic acid copolymers, tributyl acetylcitrate, simethicone, gelatin, brilliant blue FCF, black iron oxide and titanium dioxide. |
Diltiazem Sandoz CD 360 mg capsules
| Active ingredient (main ingredient) | Diltiazem hydrochloride 360 mg |
| Other ingredients (inactive ingredients) | non-pareil seed (sucrose), povidone, sodium lauryl sulfate, diethyl phthalate, purified talc, methacrylic acid copolymers, tributyl acetylcitrate, simethicone, titanium dioxide, brilliant blue FCF and gelatin |
Do not take this medicine if you are allergic to any of these ingredients.
What Diltiazem Sandoz CD Capsules looks like
Diltiazem Sandoz CD are available in bottles of 30 capsules.
All doses (180mg, 240mg and 360mg) are two component capsules:
- The 180mg capsules are blue and light blue (AUST R 131298)
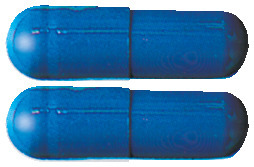
- The 240mg capsules are blue/blue (AUST R 131300)
- The 360mg capsules are light blue and white (AUST R 131304)
Who distributes Diltiazem Sandoz CD Capsules
Diltiazem Sandoz CD capsules are distributed by:
Sandoz Pty Ltd
100 Pacific Highway
North Sydney, NSW 2060
Australia
Tel 1800 726 369
This leaflet was prepared in August 2024.
diltiazem-sandoz-cd-ccdsv17-cmiv17-08aug24
Published by MIMS September 2024



 In clinical trials of diltiazem as CD capsules, tablets and SR capsules involving 3,200 patients, the most common events (i.e. greater than 1%) were oedema (4.6%), headache (4.9%), dizziness (3.5%), asthenia (2.7%), first degree AV block (2.2%), bradycardia (1.6%), flushing (1.5%), nausea (1.4%), rash (1.3%), dyspepsia (1.2%), palpitations, lower limb oedema, malaise, constipation, gastric pain and erythema.
In clinical trials of diltiazem as CD capsules, tablets and SR capsules involving 3,200 patients, the most common events (i.e. greater than 1%) were oedema (4.6%), headache (4.9%), dizziness (3.5%), asthenia (2.7%), first degree AV block (2.2%), bradycardia (1.6%), flushing (1.5%), nausea (1.4%), rash (1.3%), dyspepsia (1.2%), palpitations, lower limb oedema, malaise, constipation, gastric pain and erythema. Chemically diltiazem hydrochloride is the hydrochloride salt of (2S, 3S)-5-(2-dimethylaminoethyl)-2, 3, 4, 5-tetrahydro-2-(4-methoxyphenyl)-4-oxo-1, 5-benzothiazepin-3-yl acetate. It has a molecular weight of 450.98.
Chemically diltiazem hydrochloride is the hydrochloride salt of (2S, 3S)-5-(2-dimethylaminoethyl)-2, 3, 4, 5-tetrahydro-2-(4-methoxyphenyl)-4-oxo-1, 5-benzothiazepin-3-yl acetate. It has a molecular weight of 450.98.