What is in this leaflet
This leaflet answers some common questions about DIZOLE ONE.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have benefits and risks. Your doctor or pharmacist has weighed the risks of you taking DIZOLE ONE against the benefits expected for you.
If you have any concerns about taking this medicine, talk to your doctor or pharmacist.
Keep this leaflet with your medicine. You may need to read it again.
The information in this leaflet relates only to DIZOLE ONE. It is not to be used in relation to any other product, which may also contain the same active ingredient.
What DIZOLE ONE is used for
DIZOLE ONE is used to treat a type of fungal infection called vaginal thrush.
DIZOLE ONE contains the active ingredient fluconazole, which belongs to a group of medicines called azole antifungals. These medicines work by preventing the growth of these fungi causing your infection.
What is vaginal thrush
Vaginal thrush is a common name for vaginal candidiasis, an infection caused by a yeast-like fungus called Candida.
Candida is one of many organisms that live in the vagina. Your body's natural balance (immune system) normally keeps Candida under control, but when this natural balance is upset, Candida can multiply and can cause thrush symptoms.
Common symptoms of vaginal thrush include:
- itching, burning or soreness around the vagina
- cottage-cheese like discharge
- swelling or irritation of the infected area.
Things that may help you to avoid thrush in the future:
- wear cotton briefs, stockings and loose-fitting clothing rather than tight synthetic clothing
- wash regularly, but do not wash and dry yourself harshly
- avoid perfumed soaps, bath additives and vaginal deodorants.
Your doctor or pharmacist may have more information on things you can do to avoid thrush in the future.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
There is no evidence that DIZOLE ONE is addictive.
DIZOLE ONE is a "Pharmacist Only Medicine". It is available without a doctor's prescription but your pharmacist's advice is required.
DIZOLE ONE is not recommended for children under 18 years of age except under doctor supervision.
Before you take DIZOLE ONE
When you must not take it
Do not take DIZOLE ONE if you have an allergy to:
- any medicine containing fluconazole
- any other azole antifungals related to fluconazole such as miconazole (eg. Daktarin), ketoconazole (eg. Nizoral), clotrimazole (eg. Canestan, Clonea), or itraconazole (Sporanox)
- any of the ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin
Do not take DIZOLE ONE if you are taking
- cisapride (Prepulsid), a medicine used to treat stomach problems
- astemizole
- pimozide
- quinidine.
Combining DIZOLE ONE with the above medicines may cause serious side effects such as an abnormal heart rhythm.
Do not take DIZOLE ONE if you are pregnant, suspect you may be pregnant or if you may become pregnant during treatment. It may affect your developing baby if you take it during pregnancy.
Do not take DIZOLE ONE if you are breastfeeding. The active ingredient in DIZOLE ONE passes into breast milk and there is a possibility that your baby may be affected.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor or pharmacist.
Before you start to take it
Tell your doctor or pharmacist if you have allergies to any other medicines, foods, dyes or preservatives.
Tell your doctor or pharmacist if you have or have had any of the following medical conditions:
- thrush more than twice in the last six months
- liver problems
- kidney problems
- heart problems
- HIV infection or AIDS.
Your doctor or pharmacist may want to take special care if you have any of these conditions.
Tell your doctor or pharmacist before using DIZOLE ONE if you are taking warfarin (eg. Marevan, Coumadin), as bleeding or bruising may occur.
If you have not told your doctor or pharmacist about any of the above, tell him/her before you start taking DIZOLE ONE.
Taking other medicines
Do not take DIZOLE ONE if you are taking
- cisapride (Prepulsid), a medicine used to treat stomach problems
- astemizole
- pimozide
- quinidine.
Tell your doctor or pharmacist if you are taking any other medicines, including any that you buy without a prescription from a pharmacy, supermarket or health food shop.
Some medicines and DIZOLE ONE may interfere with each other. These include:
- warfarin (eg. Marevan, Coumadin), a medicine used to prevent blood clots
- phenytoin (e.g. Dilantin) or carbamazepine (e.g. Tegretol), medicines used to treat epilepsy
- cyclosporin (ciclosporin) (eg. Neoral), sirolimus (eg. Rapamune) or tacrolimus (eg. Prograf), medicines used to prevent organ transplant rejection or to treat certain problems with the immune system
- certain medicines use to treat diabetes such as:
- glibenclamide (eg. Daonil, Glimel), glipizide (eg. Minidiab, Melizide), glimepiride (eg. Amaryl), gliclazide (eg. Diamicron, Glyade)
- pioglitazone (eg. Actos), rosiglitazone (eg. Avandia) - rifampicin (eg. Rifadin, Rimycin) or rifabutin (eg. Mycobutin), antibiotics used to treat infections
- theophylline (eg. Nuelin), a medicine used to treat asthma
- midazolam (eg. Hypnovel) and triazolam (eg. Halcion), medicines used as sedatives or to treat anxiety
- zidovudine (eg. Retrovir), saquinavir (e.g. Invirase), medicines used to treat AIDS patients
- hydrochlorothiazide (eg. Dithiazide), a medicine used for treating fluid problems and high blood pressure
- the contraceptive pill (birth control pill)
- amphotericin B (amphotericin) (eg. Fungilin), a medicine used to treat fungal infection
- erythromycin (eg. E-Mycin) and azithromycin (e.g. Zithromax), antibiotics used to treat certain types of bacterial infections
- anticancer drugs such as cyclophosphamide monohydrate, vincristine and vinblastine, medicines used to treat certain types of cancers
- carbamazepine (eg. Tegretol), a medicine used in the treatment of epilepsy and bipolar disorder
- NSAIDS such as naproxen, diclofenac and celecoxib (eg. Celebrex)
- opioid pain killers such as alfentanil, fentanyl and methadone
- losartan, a medicine used to treat high blood pressure
- cimetidine, a medicine used to relieve heartburn and indigestion
- calcium channel blockers, such as nifedipine, amlodipine and felodipine used in relieving high blood pressure and certain heart conditions
- statins such as atorvastatin, simvastatin and fluvastatin, used to control high cholesterol levels
- antidepressants such as amitriptyline (eg. Endep) and nortriptyline.
Talk to your doctor about the need for an additional method of contraception while taking DIZOLE ONE. DIZOLE ONE may decrease the effectiveness of some birth control pills.
Your doctor can tell you what to do if you are taking any of these medicines.
These medicines may be affected by DIZOLE ONE or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
How to take DIZOLE ONE
Follow all directions given to you by your doctor and pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the pack, ask your doctor or pharmacist for help.
How much to take
For vaginal thrush in adults, only a single dose (1 capsule) of DIZOLE ONE is needed.
Do not give DIZOLE ONE to children under 18 years of age except under doctor supervision.
How to take it
Swallow the capsule whole with a glass of water.
DIZOLE ONE can be taken with or without food.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much DIZOLE ONE. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
While you are taking DIZOLE ONE
Things you must do
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking DIZOLE ONE
Tell any other doctors, dentists and pharmacists who treat you that you are taking this medicine.
Use effective contraception to prevent pregnancy while taking DIZOLE ONE.
If you become pregnant while taking this medicine, tell your doctor immediately.
If your symptoms do not improve after 3 days, tell your doctor or pharmacist.
Things you must not do
Do not take DIZOLE ONE to treat any other complaints unless your doctor or pharmacist tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Things to be careful of
Tell your doctor immediately if you develop a rash while taking DIZOLE ONE.
People with HIV, AIDS or a weak immune system may be more prone to serious side effects of the skin.
Be careful when driving vehicles or operating machinery as occasional dizziness or seizures may occur.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking DIZOLE ONE.
This medicine helps most people and is generally well tolerated. However it may have unwanted side effects in some people.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Do not be alarmed by the following list of possible side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- nausea (feeling sick), vomiting
- stomach pain, indigestion
- diarrhoea
- acne
- headache.
The above list includes the more common side effects of your medicine. They are usually mild and short-lived.
Tell your doctor as soon as possible if you notice any of the following:
- unusual muscle stiffness causing poor control of movement
- signs of frequent or worrying infections such as fever, severe chills, sore throat or mouth ulcers
- swelling of the face, lips or tongue which may cause difficulty in swallowing or breathing
- asthma, wheezing, shortness of breath
- sudden or severe itching, skin rash, hives
- fainting, seizures or fits
- flaking of the skin
- yellowing of the skin or eyes, also called jaundice
- bleeding or bruising more easily than normal, reddish or purplish blotches under the skin
- passing more urine than normal, kidney pain (pain on the sides of the body)
- symptoms of liver disease such as yellowing of the skin or eyes; dark urine, pale stools; loss of appetite; unusual tiredness
- irregular heart beat or palpitations
- increased sweating.
The above list includes serious side effects that may require medical attention. Serious side are rare.
The above list includes very serious side effects. You may need urgent medical attention or hospitalisation. These side effects are very rare
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some people.
After taking DIZOLE ONE
Storage
Keep your capsules in the pack until it is time to take them. If you take the capsules out of the pack they may not keep well.
Keep your capsule in a cool dry place where the temperature stays below 25°C.
Do not store DIZOLE ONE or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor or pharmacist tells you to stop taking this medicine or it's the expiry date has passed, ask your pharmacist what to do with it.
Product description
What it looks like
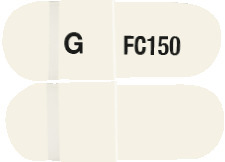
DIZOLE ONE is a hard white gelatin capsule, marked with "G" and "FC 150" in black ink.
Each pack contains 1 capsule.
Ingredients
The active ingredient in DIZOLE ONE is fluconazole 150 mg.
Each capsule also contains the following inactive ingredients:
- gelatin
- lactose monohydrate
- maize starch
- silicon dioxide
- magnesium stearate
- sodium lauryl sulfate
- titanium dioxide (E171)
- TekPrint SW-9008 Black Printing Ink
- TekPrint SW-9009 Black Printing Ink.
Dizole One contains sulfites, phenylalanine and sugars as lactose.
Supplier
DIZOLE ONE is supplied in Australia by:
Alphapharm Pty Ltd trading as Viatris
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.viatris.com.au
Phone: 1800 274 276
Australian registration number:
DIZOLE ONE - AUST R 162766
This leaflet was prepared in January 2024.
DIZOLE ONE® is a Viatris company trade mark
Published by MIMS March 2024


 Chemical name: 2-(2,4-difluorophenyl)-1, 3-bis (1H-1,2,4-triazol-1-yl)-2-propanol.
Chemical name: 2-(2,4-difluorophenyl)-1, 3-bis (1H-1,2,4-triazol-1-yl)-2-propanol.