What is in this leaflet
This leaflet answers some common questions about Erivedge capsules.
It does not contain all the available information. It does not take the place of talking to your pharmacist or doctor.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking Erivedge against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your pharmacist or doctor.
Keep this leaflet with the medicine. You may need to read it again.
What Erivedge is used for
Erivedge contains the active ingredient vismodegib.
Erivedge belongs to a group of medicines called anti-neoplastic (or anti-cancer) agents.
Erivedge is used to treat adults with a type of skin cancer called advanced basal cell carcinoma. It is used when the cancer;
- has spread to other parts of the body (called "metastatic" basal cell carcinoma) or
- has spread to surrounding areas (called "locally advanced" basal cell carcinoma) and your doctor has decided that treatment with surgery or radiation is not appropriate.
Surgery and radiation treatment may not be appropriate because;
- surgery will change the shape of a body part (cause deformity)
- with surgery, you may lose the use of a body part such as an eye or ear
- the cancer has returned after previous surgeries and further surgery isn't likely to be successful
- radiation was previously unsuccessful or you are not suitable for radiation.
Erivedge works by controlling a key protein involved in this type of cancer. Erivedge may slow or stop the cancer cells from growing, or may kill them. As a result, your skin cancer may shrink.
Research undertaken in the development of Erivedge utilised cell lines derived from human embryos.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have given it for another reason.
This medicine is not addictive.
This medicine is available only with a doctor's prescription.
Before you take it
When you must not take it
Do not take Erivedge if:
- you are pregnant, think you may be pregnant, or are planning to become pregnant during the course of treatment or during the 24 months after your final dose of the medicine.
You must not take this medicine if you are pregnant or plan to become pregnant.
Erivedge may affect your developing baby if you take it during pregnancy. Erivedge may cause your baby to die before it is born (stillborn) or cause your baby to have severe birth defects.
- you are a woman who is able to have children but you are unable or unwilling to use two acceptable forms of birth control (contraception) during the course of treatment or during the 24 months after your final dose of the medicine.
Women able to have children need to use two acceptable forms of contraception so that a pregnancy does not happen during treatment and for 24 months after the final dose.
- you are breast-feeding or intend to breast-feed in the future
The active ingredient in Erivedge may pass into breast milk and there is a possibility that your baby may suffer serious growth defects.
You must not breast-feed while taking Erivedge and for 24 months after the last dose.
- the package is torn or shows signs of tampering
If the package is damaged, return it to your pharmacist for disposal.
- the expiry date (EXP) printed on the pack has passed.
If you take this medicine after the expiry date has passed, it may not work as well.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if:
- you plan to donate blood or blood products during treatment with Erivedge or you plan to do so in the future.
If a pregnant woman receives your donated blood, the baby may develop birth defects.
You must not donate blood while taking Erivedge and for 24 months after your final dose.
- you plan to donate sperm during treatment with Erivedge or you plan to do so in the future.
If a woman receives your donated sperm, the baby may develop birth defects.
You must not donate sperm while taking Erivedge and for 2 months after your final dose.
- you have kidney problems.
- you are allergic (hypersensitive) to vismodegib or any of the ingredients listed at the end of this leaflet or allergic to any other medicines, foods, preservatives or dyes.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching, redness or hives on the skin
- chills and shivering
- feeling sick (nausea) or being sick (vomiting).
If you have not told your doctor about any of the above, tell him or her before you start taking Erivedge.
Use in Children
Erivedge should not be used in children and adolescents. The safety and effectiveness in people younger than 18 years old have not been established. Premature fusion of the growth plates has been reported in paediatric patients exposed to Erivedge. In some cases, fusion progressed after drug discontinuation.
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you have bought without a prescription from a pharmacy, supermarket or health food shop.
Tell your pharmacist or doctor if you are taking antibiotics or any herbal medicines (such as St. John's wort), because they can make female birth control (contraception) less effective.
Some medicines may be affected by Erivedge, or may affect how well it works. Your doctor will advise you about this. Your doctor or pharmacist has more information on medicines to be careful with or avoid while taking Erivedge.
Erivedge may interfere with some medicines. These include:
- ezetimibe and statins, such as atorvastatin, fluvastatin, pravastatin, rosuvastatin, simvastatin – medicines used to treat high cholesterol
- bosentan, a medicine used to treat high blood pressure in the vessels between the heart and the lungs
- glibenclamide, a medicine used to treat diabetes
- valsartan and olmesartan, medicines used to treat high blood pressure and heart problems.
Ask your doctor or pharmacist if you are not sure about any medicine you are taking.
Fertility preservation
If you are a female patient and want to have children after treatment with Erivedge, talk to your doctor about fertility preservation. It is not known whether Erivedge causes infertility. In clinical trials, some female patients no longer experienced their menstrual period after starting treatment.
How to take it
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
How much to take
The recommended dose of Erivedge is one capsule once a day.
How to take it
Swallow the capsules whole with a full glass of water.
Do not crush, open or chew the capsule.
Erivedge can be taken with or without food.
When to take it
Take Erivedge at about the same time each day. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
How long to take it
Continue taking Erivedge for as long as your doctor tells you.
How long you will be treated with Erivedge depends on how you are responding to treatment. Your doctor will discuss this with you.
If you forget to take it
If you forget to take Erivedge, do not to take the missed capsule but resume with the next scheduled dose.
Do not take a double dose to make up for the dose you missed.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering your dose, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (Australia - telephone 13 11 26; New Zealand - telephone 0800 764 766 or 0800 POISON) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much Erivedge. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
Keep telephone numbers for these places handy.
While you are taking it
Things you must do
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking Erivedge.
Tell all doctors, dentists, and pharmacists who treat you that you are taking this medicine.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking this medicine.
Both men and women who are able to have children need to take precautions so that a pregnant woman is not exposed to Erivedge. You need to do this for 24 months after stopping treatment if you are a woman and for 2 months after stopping treatment if you are a man. Erivedge may cause severe birth defects. It may also lead to the death of a baby before it is born or shortly after being born.
Talk to your doctor immediately if you have unprotected sex or if you think your contraception has failed.
Women taking Erivedge
- Women who are able to have children will need to show a negative pregnancy test (conducted under medical supervision) before starting treatment with Erivedge. You should then have a medically-supervised pregnancy test each month during treatment.
- Women who are able to get pregnant must use two forms of acceptable contraception (a barrier and a non-barrier form). This applies during treatment and for 24 months after your final dose.
- Use one non-barrier form of contraception from this list:
- combination hormonal contraceptives e.g. Yasmin, Yaz, Levlen or vaginal ring e.g. Nuvaring
- progestogen-only oral contraceptives e.g. Microlut, Provera
- subcutaneous hormonal implant e.g. Jadelle, Implanon NXT, Implanon
- birth control patch
- birth control injections e.g. Depo-Provera
- tubal sterilisation
- vasectomy (surgery performed on male partner)
- intrauterine device (IUD e.g. Copper TT38 Slimline, Multiload-Cu375, Mirena). - In addition, use one barrier form of contraception from this list:
- any male condom (with spermicide, if available)
- diaphragm (with spermicide, if available).
Talk to your doctor straight away if you become pregnant or think that you may be pregnant.
If you have stopped menstruating during the course of treatment you must still use 2 forms of acceptable contraception during treatment and for 24 months after your last dose.
Men taking Erivedge
Always use a condom (with spermicide, if available), when you have sex during treatment and for 2 months after your final dose of Erivedge.
A vasectomy does not give enough protection without a condom.
Talk to your doctor straight away if your partner becomes pregnant or thinks she is pregnant while you are taking Erivedge.
Do not donate sperm while taking Erivedge and for 2 months after your final dose.
Talk to your doctor about the best contraception for you.
Tell your doctor if, for any reason, you have not taken Erivedge exactly as prescribed. Otherwise, your doctor may think that it was not effective and change your treatment unnecessarily.
Tell your doctor if you feel Erivedge is not helping your condition.
Be sure to keep all of your appointments with your doctor so that your progress can be checked.
Things you must not do
Do not stop taking Erivedge or change the dose without first checking with your doctor.
Do not let yourself run out of medicine over the weekend or on holidays.
Do not take any other medicines whether they require a prescription or not without first telling your doctor or consulting a pharmacist.
Do not give Erivedge to anyone else even if they have the same condition as you.
Things to be careful of
Be careful driving or operating machinery until you know how Erivedge affects you. It is not known if Erivedge affects your ability to drive or operate machinery.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Erivedge.
This medicine helps most people with advanced basal cell carcinoma but it may have unwanted side effects.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by the following lists of possible side effects. You may not experience any of them.
Ask your doctor to answer any questions you may have.
Tell your doctor if you notice any of the following and they worry you:
- feeling sick, also called nausea
- diarrhoea
- constipation
- vomiting
- indigestion
- stomach pain
- feeling tired, also called fatigue
- unusual weakness
- weight loss
- decreased appetite
- a change in the way things taste or a loss of taste
- symptoms of dehydration, such as dry or sticky mouth, low or no urine output, urine looks dark yellow, no tears or sunken eyes
- muscle spasms
- pain in your chest, back, side or extremities (arms and legs)
- muscle, tendon, ligament, joint or bone pain
- aching muscles, muscle tenderness or weakness, not caused by exercise
- hair loss, also called alopecia
- loss of eyelashes
- abnormal hair growth
- loss of menstrual periods.
Erivedge may be associated with abnormalities in your blood test results.
The above list includes the more common side effects of this medicine.
Tell your doctor immediately or go to Accident and Emergency at the nearest hospital if you notice any of the following:
- shortness of breath
- difficulty breathing, chest tightness or wheezing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin.
These are very serious side effects. You may need urgent medical attention.
Tell your doctor if you notice anything that is making you feel unwell, even if it is not on this list.
This is not a complete list of all possible side effects. Others may occur in some people and there may be some side effects not yet known.
Ask your doctor or pharmacist if you don't understand anything in this list.
After using Erivedge
Storage
Keep your capsules in a cool dry place where the temperature stays below 30°C.
Keep your capsules in the bottle, with the cap tightly closed, until it is time to take them. If you take the capsules out of the bottle they may not keep well.
Do not store Erivedge or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it.
A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
Availability
Erivedge is available in a bottle of 28 capsules.
What it looks like
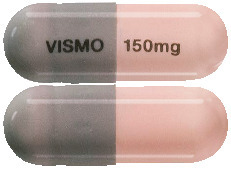
Erivedge capsules have a pink body with "150mg" printed in black ink and a grey cap with "VISMO" printed in black ink.
Ingredients
Erivedge contains 150 mg of vismodegib as the active ingredient.
It also contains the following inactive ingredients:
- microcrystalline cellulose(460)
- lactose monohydrate
- sodium lauryl sulphate
- povidone
- sodium starch glycollate
- purified talc(553)
- magnesium stearate
The capsule shell contains:
- gelatin
- titanium dioxide (171)
- iron oxide red (CI77491, 172)
- iron oxide black (CI77499, 172)
- shellac (904)
This medicine does not contain, sucrose, gluten, tartrazine or any other azo dyes.
Manufacturer
Erivedge is distributed in Australia by:
Roche Products Pty Ltd
ABN 70 000 132 865
Level 8, 30 – 34 Hickson Road
Sydney NSW 2000
AUSTRALIA
Medical enquiries: 1800 233 950
Distributed in New Zealand by:
Roche Products (New Zealand) Limited
PO Box 109113 Newmarket
Auckland 1149
NEW ZEALAND
Medical enquiries: 0800 656 464
Please check with your pharmacist for the latest Consumer Medicine Information.
Australian Registration Number:
- AUST R 214475
This leaflet was prepared 06 April 2020
Published by MIMS May 2020




 The waterfall plots in Figures 1 and 2 represent IRF assessment at 12 month follow-up by charting the maximum reduction in target lesion(s) size for each patient. The majority of patients in both cohorts experienced tumour shrinkage.
The waterfall plots in Figures 1 and 2 represent IRF assessment at 12 month follow-up by charting the maximum reduction in target lesion(s) size for each patient. The majority of patients in both cohorts experienced tumour shrinkage.
 At the time of the primary analysis for mBCC the majority of IRF assessed responses (6 of 10 responders) occurred by week 8 and additional responses were observed at later assessments. For laBCC the majority of IRF assessed responses (14 of 27 responders) occurred by week 8 and additional responses were observed at later assessments. 54% of laBCC patients (n = 63) had a histopathologic response with no evidence of BCC at 24 weeks.
At the time of the primary analysis for mBCC the majority of IRF assessed responses (6 of 10 responders) occurred by week 8 and additional responses were observed at later assessments. For laBCC the majority of IRF assessed responses (14 of 27 responders) occurred by week 8 and additional responses were observed at later assessments. 54% of laBCC patients (n = 63) had a histopathologic response with no evidence of BCC at 24 weeks.
