What is in this leaflet
This leaflet answers some common questions about EUTROXSIG tablets.
It does not contain all the available information about the medicine.
It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking EUTROXSIG against the benefits they expect it will have.
Ask your doctor or pharmacist if you have any concerns about taking this medicine.
Keep this leaflet with the medicine. You may need to read it again.
What is EUTROXSIG used for
EUTROXSIG is available in tablets of four different strengths: 50 micrograms, 75 micrograms, 100 micrograms and 200 micrograms.
The active ingredient in EUTROXSIG is levothyroxine sodium, which is a thyroid hormone. It is used as replacement therapy in the treatment of thyroid hormone deficiency.
EUTROXSIG is used to treat:
- Thyroid hormone deficiency also known as hypothyroidism.
Hypothyroidism is a disease in which the thyroid gland is underactive and does not produce enough thyroxine, a hormone, which is important for controlling your metabolism. Symptoms of hypothyroidism include tiredness, muscle weakness and cramps; feeling the cold; a slow heart rate; dry and flaky skin; hair loss; a deep husky voice and weight gain. - TSH-responsive tumours (certain tumours of the thyroid gland) of the thyroid.
For these conditions to be treated, patients need a supply of thyroid hormones in their body. EUTROXSIG replaces the shortage of thyroid hormones.
Use EUTROXSIG only as directed.
Your doctor may have prescribed the medicine for another condition.
Ask your doctor if you have any questions about why it has been prescribed for you.
This medicine is only available with a doctor’s prescription.
Before you take it
When you must not take it
Do not take EUTROXSIG if you are allergic to:
- levothyroxine sodium or any other thyroid hormone (e.g. Tertroxin)
- any of the inactive ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction to EUTROXSIG may include red, itchy skin rashes; difficulty in breathing; swelling of the face or throat or faintness.
Do not use it after the expiry date (EXP.) printed on the pack. If you take the medicine after the expiry date has passed, it may not work as well.
Do not take it if the packaging shows signs of tampering.
Before you start to take it
Tell your doctor if:
- you are allergic to any other medicines or any foods, dyes or preservatives.
- you are pregnant or intend to become pregnant.
Thyroxine levels will need to be watched carefully during pregnancy. Your dosage of EUTROXSIG may need to be increased while you are pregnant.
Ask your doctor about the risks and benefits of taking it during pregnancy. - you are breastfeeding or intend to breastfeed.
Although small amounts of the medicine are found in breast milk, women who are breastfeeding should continue treatment with EUTROXSIG. - you have or have had any other medical conditions or health problems, including:
- overactive thyroid gland
- adrenal gland problem
- hyperthyroidism
- heart problems such as cardiovascular disorder
- high blood pressure
- diabetes
- long-standing hypothyroidism, an underactive thyroid gland.
- problems absorbing nutrients from the gastrointestinal tract
If you have not told your doctor about any of the above, tell them before you start to take any EUTROXSIG.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including medicines that you buy without a prescription from a pharmacy, supermarket or health food shop.
Some of these medicines may interfere with EUTROXSIG. These include:
- anticoagulants, medicines used to thin your blood (e.g. Warfarin)
- antidepressants, medicines used to treat depression (e.g. lithium, SSRIs, tricyclic antidepressants)
- antivirals, medicines used to treat HIV/AIDS infection (e.g. ritonavir)
- antimalarials, medicines used to treat and prevent malaria (e.g. chloroquine and proguanil)
- medicines used to treat diabetes (e.g. insulin)
- beta-blockers, medicines used to treat high blood pressure and heart conditions (e.g. propranolol)
- ion-exchange resins, medicines used to decrease cholesterol in the blood (e.g. cholestyramine)
- corticosteroids, anti inflammatory medicines (e.g. prednisolone and dexamethasone)
- oral contraceptives and hormone replacement medicines such as oestrogens and androgens
- medicines used for epilepsy (e.g. phenytoin and carbamazepine)
- medicines used to treat heart failure (e.g. digoxin)
- iron supplements
- calcium supplements
- rifampicin, an antibiotic used to treat tuberculosis and other serious infections
- ciprofloxacin, an antibiotic used to treat various infections
- soyabean flour (e.g. some infant formula and other products)
- antacids (e.g. aluminium hydroxide, magnesium hydroxide and calcium carbonate)
- amiodarone, a medicine used to treat irregular heart beat
- oral contrast agents, used before X-ray and scans
- propylthiouracil, a medicine used to treat overactive thyroid and Graves disease
- non-steroidal anti-inflammatory drugs (NSAIDs), medicines used to relieve pain and/or inflammatory conditions including arthritis.
- weight loss drugs (e.g. orlistat)
- tyrosine kinase inhibitors, medicines used to treat cancer (ie imatinib and sunitinib)
- statins, medicines used to lower cholesterol
- tamoxifen and 5-flourouracil, medicines used to treat tumours
- methadone (a narcotic)
- St John’s wort, a herbal medicine
- biotin (also known as Vitamin H, Vitamin B7 or Vitamin B8)
These medicines may affect how well EUTROXSIG works or react with it resulting in unwanted or sometimes serious side effects.
This list is not exhaustive. Your doctor or pharmacist has more information on medicines to be careful with or avoid while taking it.
How to take it
It may take a few weeks for EUTROXSIG to begin working. Until it begins working you may not notice any change in your symptoms.
Follow your doctor's instructions carefully, as they may differ from the information contained in this leaflet.
If you do not understand the instructions on the blister, ask your doctor or pharmacist for help.
How much to take
Carefully follow the dosage instructions, as given by your doctor.
The usual starting dose for adults is 50 to 100 micrograms daily. The dose may be increased over time. The average adult maintenance dose is 100 to 200 micrograms. Lower doses are used in the elderly and children. Your doctor will calculate the dose required for you.
Your doctor will monitor your blood tests to make sure EUTROXSIG is working for you.
Do not change your dose of EUTROXSIG unless your doctor tells you to do so.
Talk to your doctor if you have any further questions.
How to take it
Swallow EUTROXSIG tablets with a glass of water.
When to take it
EUTROXSIG tablets should be taken first thing in the morning on an empty stomach, at least 30 minutes and preferably 60 minutes before any food or other medications.
How long to take it
Continue taking EUTROXSIG as long as your doctor recommends it.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take your dose as soon as you remember, and then go back to taking it as you would normally.
Do not take a double dose to make up for the dose that you missed.
If you are unsure about whether to take your next dose, speak to your doctor or pharmacist.
If you have trouble remembering when to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at your nearest hospital, if you think that you or anyone else may have taken too much EUTROXSIG. Do this even if there are no signs of discomfort or poisoning.
Symptoms of overdose include restlessness; vomiting; flushing; breathing difficulties; chest pain; convulsions or paralysis.
While you are taking it
Things you must do
Do not switch or interchange with other brands unless advised by your doctor.
Immediately stop taking EUTROXSIG if a skin rash or other allergic reaction occurs.
Use it exactly as your doctor has prescribed.
Tell your doctor if you feel the medicine is not helping your condition.
Visit your doctor regularly. Your doctor needs to check your progress and see whether you need to stop taking EUTROXSIG.
Tell any other doctors, dentists and pharmacists who are treating you that you are taking EUTROXSIG.
Always discuss with your doctor any problems or difficulties during or after taking the medicine.
If you plan to have surgery, tell your doctor or dentist that you are taking EUTROXSIG.
If you are about to start taking any new medicines, remind your doctor and pharmacist that you are taking EUTROXSIG.
Ensure you do not run out of medicine over the weekend or on holidays.
Things you must not do
Do not drive or operate machinery where alertness is required, until you know how it affects you.
Do not give this medicine to anyone else, even if his or her symptoms seem similar to yours.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking EUTROXSIG.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- nervousness
- anxiousness
- excitation
- restlessness
- muscle weakness & cramps
- inability to sleep
- sleep disturbances
- unusual movements, including tremor
- headache
- lack of concentration
- diarrhoea
- stomach cramp
- nausea
- vomiting
- heat intolerance
- excessive sweating
- flushing
- weight loss
- menstrual irregularities
- decreased libido
- fever
- shortness of breath
- rapid breathing
- irregular heart beats
- chest pain
- increased blood pressure
- allergic reactions such as skin rash
- hair loss
- irritability.
- increased appetite
- tiredness
Some people may get other side effects with EUTROXSIG.
Check with your doctor as soon as possible if you have any problems while taking EUTROXSIG even if you do not think the problems are connected with this medicine or are not listed in this leaflet.
Do not be alarmed by the above list of possible side effects. You may not experience any of them.
After taking it
Storage
Store at 2°C to 8°C (Refrigerate. Do not freeze).
Always store tablets in their original blister strips. If you do not keep the tablets in the blister strip they may not keep as well.
Laboratory tests have shown that if not stored correctly, there is a reduction in potency of the active ingredient levothyroxine sodium.
In-use blisters:
A single blister strip can be removed from the carton and stored below 25°C for up to 14 days (2 weeks). After 14 days (2 weeks) of storage below 25°C, discard any remaining tablets.
Where unavoidable (e.g. in warm climates where temperatures regularly exceed 25°C), an in-use blister strip may continue to be stored in a refrigerator at 2°C to 8°C (Refrigerate. Do not freeze) for up to 14 days (2 weeks).
EUTROXSIG tablets can also be stored in Webster packs for 14 days (2 weeks) below 25°C.
Do not store this medicine or any other medicines in a bathroom or near a sink. Do not leave it in the car or on windowsills. Heat and dampness can destroy some medicines.
Do not take EUTROXSIG tablets if the tablet’s colour has changed.
Keep the medicine out of reach of children.
Disposal
If your doctor tells you to stop taking this medicine or it has passed its expiry date, ask your pharmacist what to do with any left over.
Product description
What it looks like
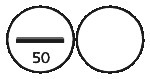
EUTROXSIG 50 microgram tablets are white, biconvex, round tablets, scored and marked “50” on one side, plain on the other side.
EUTROXSIG 75 microgram tablets are white, biconvex, round tablets, scored and marked “75” on one side, scored on the other side.
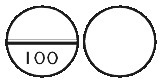
EUTROXSIG 100 microgram tablets are white, biconvex, round tablets, scored and marked “100” on one side, plain on the other side.
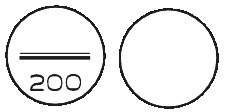
EUTROXSIG 200 microgram tablets are white, biconvex, round tablets, scored and marked “200” on one side, plain on the other side.
EUTROXSIG tablets are available in blister packs.
Each pack contains 200 tablets.
Ingredients
Active ingredient:
Each EUTROXSIG tablet contains levothyroxine sodium as the active ingredient.
Inactive ingredients:
- maize starch
- lactose monohydrate
- dextrin
- magnesium stearate.
EUTROXSIG tablets are free from gluten, sucrose and azo dyes.
Sponsor
Sponsored in Australia by:
Aspen Pharma Pty Ltd
34-36 Chandos St
St Leonards NSW 2065
The Australian Registration Numbers for EUTROXSIG tablets are:
- 50 micrograms; AUST R 125501
- 75 micrograms; AUST R 144117
- 100 micrograms; AUST R 125502
- 200 micrograms; AUST R 125503.
This leaflet was revised in January 2025.
Published by MIMS April 2025