What is in this leaflet
This leaflet answers some common questions about Exelon.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
The information in this leaflet was last updated on the date listed on the final page. More recent information on the medicine may be available.
You should ensure that you speak to your pharmacist or doctor to obtain the most up to date information on the medicine. You can also download the most up to date leaflet from www.novartis.com.au. Those updates may contain important information about the medicine and its use of which you should be aware.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking it against the benefits they expect it will provide.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What Exelon is used for
Exelon is used to treat a condition called Alzheimer's disease.
Alzheimer's disease is a condition in which changes in the brain cause problems with memory, thinking and behaviour. These problems gradually become worse with time.
Exelon works by preventing the breakdown of a chemical in the brain called acetylcholine. This chemical is needed to help keep the brain working properly.
Exelon helps to slow down the mental decline that happens in people with Alzheimer's disease and it helps to improve the ability to cope with everyday activities. It does not cure the condition.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
Exelon is only available with a doctor's prescription. It is not addictive.
There is not enough information to recommend this medicine for children.
Before you take Exelon
When you must not take it
Do not take Exelon if you have had an allergic reaction to any of the following:
- rivastigmine, the active ingredient in Exelon
- any of the other ingredients of Exelon listed at the end of this leaflet.
- other related "carbamate" medicines (if you are unsure about these, ask your doctor or pharmacist)
Symptoms of an allergic reaction may include wheezing or difficulty breathing; swelling of the face, lips, tongue or other parts of the body; rash or hives on the skin.
Do not take Exelon if you have a severe liver disorder. There is no information on the use of this medicine in people with severe liver problems.
Do not take Exelon if you have had a skin reaction spreading beyond Exelon transdermal patch size, if there was a more intense local reaction (such as blisters, increasing skin inflammation, swelling) and if it did not improve within 48 hours after removal of the transdermal patch.
Do not take Exelon after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. In that case, return it to your pharmacist.
Before you start to take it
Tell your doctor if you have any of the following conditions:
- a problem with your heart including an irregular or slow heartbeat, QTc prolongation, a family history of QTc prolongation, torsade de pointes, or family history of low potassium or magnesium
- a history of stomach ulcer
- problems with your lungs such as asthma or emphysema
- difficulty passing urine (water)
- seizures (fits)
- problems with your kidneys or liver
- problems with your stomach such as nausea (feeling sick) and vomiting (being sick)
- if you have a low body weight (less than 50 kg)
If you have any of the above conditions your doctor may want to take special precautions while you are taking this medicine.
Tell your doctor if you are pregnant or breast-feeding. It is not known whether taking Exelon during pregnancy or while breast-feeding could affect your baby. Breast-feeding is not recommended while you are taking this medicine.
Tell your doctor if you smoke. Nicotine can affect the amount of Exelon that is in your body. A sudden change in your usual smoking habit can also change the effects of Exelon.
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you buy without a prescription from a pharmacy, supermarket or health food shop.
Some medicines and Exelon may interfere with each other. These include:
- cholinergic medicines (e.g. bethanecol, medicines used during surgery)
- anticholinergic medicines (e.g. medicines for stomach cramps, medicines for travel sickness, many medicines used to treat mental illness)
- non-steroidal anti-inflammatory drugs (NSAIDs), which are medicines used to treat arthritis and other painful conditions such as muscle strains, back pain, menstrual cramps and migraine
- metoclopramide, medicine used for nausea and vomiting.
- beta-blockers (e.g. atenolol), medicines used to treat hypertension, angina and other heart conditions
Use caution when taking Exelon together with medicinal products know to prolong the heart’s electrical system (QT interval). This could be including drugs like quinidine, amiodarone, pimozide, halofantrine, cisapride, citalopram, mizolastin, moxifloxacin, erythromycin, chlorpromazine, levomepromazine, sulpiride, sultopride, amisulpride, tiapride, veralipride, haloperidol, droperidol, diphemanil, methadone, and pentamidine. Your doctor may also monitor your clinical condition as needed.
You may need to take different amounts of your medicines or to take different medicines while you are taking Exelon. Your doctor and pharmacist have more information.
If you have not told your doctor about any of these things, tell him/her before you start taking this medicine.
How to take Exelon
Always take this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist, or healthcare provider if you are not sure. These instructions may differ from the information contained in this leaflet.
If you do not understand the instructions on the label, ask your doctor or pharmacist for help.
How much to take
The usual starting dose is 1.5 mg twice a day. After two weeks, if you do not have any problems with the medicine, the dose may be gradually increased up to a maximum of 6 mg twice a day.
If for any reason you stop taking Exelon for more than three days, tell your doctor before you start taking Exelon again. Your doctor will restart you at the lowest dose to help prevent side effects such as nausea and vomiting.
How to take it
Exelon is available in capsules.
If you are taking the capsules, swallow them whole with a full glass of water or other liquid, with your morning and evening meals. Do not open or crush the capsules.
Take Exelon at about the same time each day. Taking your medicine at the same time each day will have the best effect. It will also help you remember when to take it.
How long to take it
Continue taking Exelon for as long as your doctor tells you to. This medicine helps to slow the progression of Alzheimer's disease but does not cure it. Your treatment can be continued for as long as it benefits your condition. Your doctor can give you more information.
Do not stop taking Exelon or change your dose without talking with your doctor.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and take the next dose when you are meant to.
Otherwise, take it as soon as you remember, and then go back to taking it as you would normally.
Do not take a double dose to make up for the one that you missed. This may increase the chance of you getting an unwanted side effect.
If you have trouble remembering when to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone number 13 11 26), or go to Accident and Emergency at your nearest hospital if you think that you or anyone else may have taken too much Exelon. Do this even if there are no signs of discomfort or poisoning.
Keep the telephone numbers for these places handy.
Some of the symptoms of an overdose may include severe nausea (feeling sick), vomiting, diarrhoea, slow heartbeat and breathing, excess saliva, sweating, muscle weakness, fainting and seizures (fits).
While you are taking Exelon
Things you must do
Be sure to keep all of your doctor's appointments so your progress can be checked.
You and your caregiver can help to produce the maximum benefit from your treatment by keeping in close contact with your doctor.
Make sure you or your caregiver tells your doctor if you experience considerable nausea, vomiting or diarrhoea with loss of appetite and weight loss. You may become dehydrated (losing too much fluid) if vomiting or diarrhoea are prolonged.
Talk to your doctor right away if you have skin inflammation, blisters or swelling of the skin that are increasing and spreading.
If you become pregnant while taking Exelon, tell your doctor.
If you are going to have surgery, tell your doctor and anaesthetist that you are taking Exelon. Exelon may affect some medicines you receive during surgery.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking Exelon.
Tell any other doctor, dentist or pharmacist who treats you that you are taking Exelon.
Things you must not do
Do not give this medicine to anyone else even if their condition seems similar to yours.
Do not use Exelon to treat any other complaints unless your doctor tells you to.
Your doctor will tell you whether your illness allows you to drive vehicles and use machines safely. Exelon may cause dizziness and somnolence, mainly at the start of treatment or when increasing the dose. Therefore, you should wait to know what effects the drug may cause before engaging in such activities. If you feel dizzy or drowsy, do not drive, use machines or perform any other tasks that require your attention.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Exelon, even if you do not think it is connected with the medicine.
All medicines can have side effects. Sometimes they are serious, but most of the time they are not. You may need medical treatment if you get some of the side effects.
Do not be alarmed by these lists of possible side effects. You may not experience any of them. Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you notice any of the following and they worry you:
- nausea (feeling sick), vomiting
- diarrhoea
- loss of appetite
- indigestion, abdominal discomfort or stomach pain
- weight loss
- dizziness
- headache
- itching
- unusual tiredness, weakness or sleepiness, feeling generally unwell
- trembling or shakiness
- increased sweating
- difficulty sleeping
- nightmares
- confusion
- feeling anxious, agitated or depressed
- aggression
- restlessness, hyperactivity
- hallucinations (hearing or seeing things that are not there)
- loss of control of your bladder or bowels (incontinence).
The above side effects usually happen at the start of treatment when the dose is being increased. They are not usually serious and may gradually disappear as your body gets used to the medicine.
Women are more likely than men to get some side effects (e.g. nausea, vomiting, loss of appetite, weight loss).
Tell your doctor immediately or go to Accident and Emergency at your nearest hospital if you notice any of the following:
- signs of allergy such as rash or hives on the skin; swelling of the face, lips, tongue or other parts of the body; wheezing or difficulty breathing
- chest pain
- unusually fast, slow or irregular heart beat
- severe dizziness, fainting or fits (seizures)
- severe confusion, loss of coordination, difficulty speaking or breathing
- vomiting blood or material that looks like coffee grounds
- bleeding from the back passage, black sticky bowel motions (stools) or bloody diarrhoea
- severe pain in the abdomen, often with nausea and vomiting
- signs of a urinary tract infection such as frequent urge to urinate or pain on urination
- signs of a liver disorder (yellow skin, yellowing of the whites of eyes, abnormal darkening of the urine or unexplained nausea, vomiting, tiredness and loss of appetite)
- skin inflammation, blisters or swelling of the skin that are increasing and spreading
- dehydration (losing too much fluid)
- hallucinations
- stiff limbs, trembling hands.
Tell your doctor if you notice anything else that is making you feel unwell. Some people may have other side effects not yet known or mentioned in this leaflet. Some of the side effects (for example, changes in liver function) can only be found when your doctor does tests from time to time to check your progress.
After using Exelon
Storage
- Keep your medicine in the original container until it is time to take it.
- Store the medicine at room temperature.
- Do not store Exelon or any other medicine in the bathroom or near a sink.
- Do not leave it in the car or on window sills.
Keep the medicine where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking Exelon or the expiry date has passed, ask your pharmacist what to do with any medicine you have left over.
Product description
What it looks like
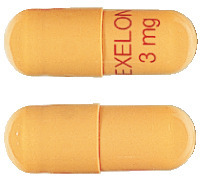
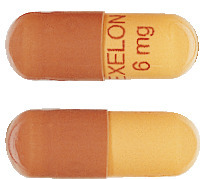
Exelon capsules are available in four strengths, in packs of 56 capsules.
- Exelon 1.5 mg: yellow capsules printed with "EXELON" and "1.5 mg" in red.
- Exelon 3.0 mg: orange capsules printed with "EXELON" and "3 mg" in red.
- Exelon 4.5 mg: red capsules printed with "EXELON" and "4.5 mg" in white.
- Exelon 6.0 mg: orange and red capsules printed with "EXELON" and "6 mg" in red
Ingredients
Exelon capsules contain 1.5, 3.0, 4.5 or 6.0 mg of the active ingredient, rivastigmine (as the hydrogen tartrate salt). They also contain:
- magnesium stearate
- hypromellose
- colloidal anhydrous silica
- microcrystalline cellulose
- gelatin
- red iron oxide (CI77491)
- yellow iron oxide (CI77492)
- titanium dioxide
- printing ink (containing red iron oxide or titanium dioxide, and shellac).
Possible allergens: capsules contain tartrazine and residual sulfites, phenylalanine.
Sponsor
Exelon is supplied in Australia by:
NOVARTIS Pharmaceuticals Australia Pty Limited
ABN 18 004 244 160
54 Waterloo Road
Macquarie Park NSW 2113
Telephone 1 800 671 203
®= Registered Trademark
This leaflet was prepared in August 2023
Australian Registration Number.
1.5 mg capsule AUST R 71445
3.0 mg capsule AUST R 71446
4.5 mg capsule AUST R 71447
6.0 mg capsule AUST R 71448
(exe280823c) based on PI (exe280823i)
Published by MIMS October 2023



 Other adverse events observed at a rate of 2% or more on Exelon 6-12 mg/day but at a greater or equal rate on placebo were chest pain, peripheral oedema, vertigo, back pain, arthralgia, pain, bone fracture, agitation, nervousness, delusion, paranoid reaction, upper respiratory tract infections, infection (general), coughing, pharyngitis, bronchitis, rash (general), urinary incontinence, nightmares.
Other adverse events observed at a rate of 2% or more on Exelon 6-12 mg/day but at a greater or equal rate on placebo were chest pain, peripheral oedema, vertigo, back pain, arthralgia, pain, bone fracture, agitation, nervousness, delusion, paranoid reaction, upper respiratory tract infections, infection (general), coughing, pharyngitis, bronchitis, rash (general), urinary incontinence, nightmares.


