What is in this leaflet
This leaflet answers some common questions about EXFORGE.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
The information in this leaflet was last updated on the date listed on the final page. More recent information on the medicine may be available.
You should ensure that you speak to your pharmacist or doctor to obtain the most up to date information on the medicine. You can also download the most up to date leaflet from www.novartis.com.au. Those updates may contain important information about the medicine and its use of which you should be aware.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking this medicine against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What EXFORGE is used for
EXFORGE is used to control high blood pressure, also called hypertension.
Everybody has blood pressure. This pressure helps get your blood all around your body. Your blood pressure may be different at different times of the day, depending on how busy or worried you are. You have hypertension (high blood pressure) when your blood pressure stays higher than is needed, even when you are calm and relaxed.
High blood pressure increases the workload of the heart and blood vessels. If it continues for a long time, it can damage the blood vessels in the brain, heart and kidneys. This can lead to stroke, heart failure or kidney failure. High blood pressure increases the risk of heart attacks. Lowering your blood pressure reduces the chance of these disorders happening.
EXFORGE contains valsartan and amlodipine besylate. These medicines reduce blood pressure in two different ways.
- Valsartan blocks the effect of angiotensin II, which is a substance in the body that tightens blood vessels and makes your blood pressure rise. When the effect of angiotensin II is blocked, your blood vessels relax and your blood pressure goes down.
- Amlodipine besylate blocks the movement of calcium into the cells of the heart and blood vessels. As a result, they relax blood vessels and increase the supply of blood and oxygen to the heart while reducing its workload.
EXFORGE is used to treat high blood pressure in patients whose blood pressure is not controlled enough with either amlodipine or valsartan on its own.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another purpose.
There is not enough information to recommend the use of EXFORGE in children.
This medicine is only available with a doctor's prescription. It is not addictive.
Before you take EXFORGE
When you must not take it
Do not take EXFORGE if you have ever had an allergic reaction after taking:
- valsartan or amlodipine besylate (the active ingredients in EXFORGE)
- medicines belonging to a group of chemicals called dihydropyridines, used to treat blood pressure and other heart problems.
- any of the other ingredients listed at the end of this leaflet
Symptoms of an allergic reaction may include shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue or other parts of the body; rash, itching or hives on the skin.
Do not take EXFORGE if you are pregnant or intend to become pregnant. EXFORGE is not recommended for use in pregnancy. Like other similar medicines, it could affect your unborn baby.
Do not take EXFORGE if you have any of the following medical conditions:
- severe kidney disease or are having dialysis
- severe liver disease including biliary cirrhosis
- cholestasis, which is reduced or stopped bile flow
Do not take Exforge if you are also taking other blood pressure lowering medicines containing aliskiren and have type 2 diabetes.
Do not take EXFORGE after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. In that case, return it to your pharmacist.
Before you start to take it
Tell your doctor if you have/have had any of the following health problems/medical conditions:
- severe kidney problems or received a kidney transplant
- liver problems
- heart problems, including obstructed blood flow from narrowing of valves (stenosis) or enlarged septum (HOCM)
- a condition known as primary hyperaldosteronism (Conn's syndrome), a hormone disorder causing fluid retention
- swelling, mainly of the face and throat, while taking other medicines (including an ACE inhibitor or aliskiren)
- suffering from several episodes of vomiting or diarrhoea or are taking a diuretic (a drug increasing the amount of urine).
Your doctor may want to take special precautions if you have any of the above conditions.
Tell your doctor if you are breast-feeding a baby. Ask your doctor about the risks and benefits of taking EXFORGE in this case. It is not known if valsartan or amlodipine, the active ingredients of EXFORGE, pass into the breast milk and could affect your baby.
Tell your doctor if you are allergic to any other medicines, foods, dyes or preservatives. Your doctor will want to know if you are prone to allergies.
If you have not told your doctor about any of these things, tell him/her before you take EXFORGE.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including medicines that you buy without a prescription from a pharmacy, supermarket or health food shop.
Some medicines and EXFORGE may interfere with each other. Your doctor may need to change the dose or take other precautions. In some cases you may have to stop taking one of the medicines. This applies especially to the medicines listed below:
These include:
- medicines used to treat high blood pressure and some other heart conditions including fluid tablets or diuretic medicines, ACE-inhibitors, aliskiren and beta blockers
- simvastatin (a medicine used to help lower cholesterol levels), since the dose may have to be reduced when taken with Exforge
- some antibiotics (rifampicin), anti-rejection drugs (ciclosporin), antiretrovirals (ritonavir) which may increase the effect of valsartan
- tablets, preparations or supplements which increase the potassium levels in your blood (such as certain types of diuretics, potassium supplements, salt substitutes etc), lithium (a medicine used to treat some types of depression)
- anticonvulsant agents (e.g. carbamazepine, phenobarbital, phenytoin, fosphenytoin, primidone), rifampicin, St. John's wort
- nitroglycerin and other nitrates, or other substances called "vasodilators"
- medicines used for HIV/AIDS (e.g. ritonavir) or for treatment of fungal infections (e.g. ketoconazole)
- certain types of pain killers called non-steroidal anti-inflammatory medicines (NSAIDs) or Selective Cyclooxygenase-2 Inhibitors (Cox-2 Inhibitors).
- intravenous dantrolene used to treat malignant hyperthermia
Your doctor may also check your kidney function.
Your doctor and pharmacist have a more complete list of medicines to be careful of while taking EXFORGE.
How to take EXFORGE
Follow carefully all directions given to you by your doctor and pharmacist. These instructions may differ from the information contained in this leaflet.
If you do not understand the instructions on the label, ask your doctor or pharmacist for help.
How much to take
Your doctor will tell you how many tablets to take each day. The usual dose is one tablet a day.
Depending on how you respond to the treatment, your doctor may suggest a higher or lower dose.
When to take it
Take your EXFORGE dose at the same time each day. This also helps you remember to take it, especially if you take it as part of your usual routine (e.g. at breakfast time). This medicine will keep working for the whole 24 hours until the next dose is due.
How to take it
Swallow the tablet with a full glass of water.
Always take EXFORGE in the same way, with or without food. You can take it with or without food but it will work best if you always take it in the same way every day.
How long to take it
Take this medicine until your doctor tells you to stop even if you feel quite well. People who have high blood pressure often feel well and do not notice any signs of this problem.
If you forget to take it
If it is almost time for your next dose, skip the missed dose and take the next one when you are meant to.
Otherwise, take the dose as soon as you remember and then go back to taking it as you would normally.
Do not take a double dose to make up for the one that you missed. This may increase the chance of side effects.
If you have trouble remembering when to take your medicine, ask your pharmacist for some hints.
If you take too much (Overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone number: 13 11 26) for advice, or go to Accident and Emergency at your nearest hospital if you think that you or anyone else may have taken too much EXFORGE. Do this even if there are no signs of discomfort or poisoning. Keep the telephone numbers for these places handy. Too much EXFORGE may make you feel dizzy, light headed or faint. You may experience rapid, shallow breathing or cold, clammy skin. Your heartbeat may be faster than usual. This is because your blood pressure is too low.
While you are taking EXFORGE
Things you must do
If you become pregnant while taking EXFORGE, tell your doctor immediately. You should not take this medicine while you are pregnant.
Tell your doctor if, for any reason, you have not taken your medicine exactly as prescribed. Otherwise your doctor may think that it was not effective and change your treatment unnecessarily.
Be sure to keep all of your doctor's appointments so that your progress can be checked. Do this even if you feel well. People with high blood pressure often do not notice any signs of this problem. But it is important to keep track of your progress. Your doctor will want to check your blood pressure and your kidney and liver function from time to time.
If you are going to have surgery, tell your doctor and anaesthetist that you are taking EXFORGE. EXFORGE may affect some medicines you receive during surgery.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking EXFORGE.
Tell any other doctor, dentist or pharmacist who treats you that you are taking EXFORGE.
Things you must not do
Do not use EXFORGE to treat any other complaints unless your doctor says you can.
Do not give this medicine to anyone else, even if their condition seems to be similar to yours.
Things to be careful of
Avoid eating grapefruit or drinking grapefruit juice. Grapefruit juice can affect the metabolism of some medicines, including amlodipine.
Be careful driving, operating machinery or doing jobs that require you to be alert while you are taking EXFORGE until you know how it affects you. This medicine can cause tiredness, sleepiness or dizziness in some people. If you have these symptoms, do not drive or do anything else that could be dangerous.
If this medicine makes you feel dizzy or light-headed, be careful when getting up from a sitting or lying position. Dizziness can usually be prevented by getting up slowly and flexing leg muscles and toes to get the blood flowing. When getting out of bed, dangle your legs over the side for a minute or two before standing up.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking EXFORGE, even if you do not think it is connected with the medicine.
All medicines can have side effects. Sometimes they are serious, but most of the time they are not. You may need medical treatment if you get some of the side effects.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you notice any of these side effects and they worry you:
- headache
- buzzing, whistling, ringing or other persistent noise in the ears
- dizziness, spinning sensation, changes in vision, uncoordinated movements
- dizziness on standing up, especially when getting up from a sitting or lying position
- sleepiness, tiredness, weakness or difficulty sleeping
- pain in the back or joints
- muscle pain, muscle tenderness or weakness, cramps
- runny nose or congested sinuses
- dry cough, sore throat or hoarse voice
- dry mouth
- bleeding, tender or enlarged gums
- stomach upset, pain, diarrhoea or constipation
- nausea (feeling sick) or vomiting
- tingling or numbness in hands or feet
- rash, redness, blistering or peeling of skin, itching
- excessive sweating
- feeling anxious or sad
- problems with sexual function
- breast enlargement in men
- unusual hair loss or thinning
- passing more urine than normal or frequent urge to urinate
- redness and warm feeling of the face and/or neck
- swelling of the ankles, feet, face or hands
- flushing
- palpitations
- indigestion
- unusual tiredness or weakness
- weight gain
- feeling nervous, depressed or moody
- distorted sense of taste
- sensitivity to light
Tell your doctor immediately or go to Accident and Emergency at your nearest hospital if you notice any of the following:
- signs of allergy such as rash, itching or hives on the skin; swelling of the face, lips, tongue or other parts of the body; shortness of breath, wheezing or troubled breathing
- feeling of fast or irregular heart beat (pounding, racing, skipping beats)
- chest pain
- tiredness or lack of energy, being short of breath when exercising
- bleeding or bruising more easily than normal
- constant "flu-like" symptoms such as chills, fever, sore throat, aching joints, sores in mouth, swollen glands
- severe dizziness or fainting
- stomach pain with nausea, vomiting, loss of appetite, feeling generally unwell, fever, itching, yellowing of the skin and eyes, and dark coloured urine
The above list includes serious side effects which may require medical attention. These side effects do not occur frequently.
Tell your doctor if you notice anything else that is making you feel unwell. Other side effects not listed above may also occur in some people.
After using EXFORGE
Storage
- Keep your tablets in the original container until it is time to take them.
- Store them in a cool dry place below 30°C (room temperature).
- Do not store EXFORGE or any other medicine in the bathroom or near a sink.
- Do not leave EXFORGE in the car or on window sills.
Heat and dampness can destroy some medicines. EXFORGE will keep well if it is cool and dry.
Keep medicines where children cannot reach them. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking EXFORGE, or it has passed its expiry date, ask your pharmacist what to do with any tablets that are left over.
Product description
What it looks like
EXFORGE 5/80 (5 mg of amlodipine and 80 mg valsartan) tablets are dark yellow, round and imprinted with NVR on one side and NV on the other. Packs of 7 and 28.
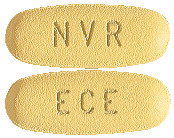
EXFORGE 5/160 (5 mg of amlodipine and 160 mg valsartan) tablets are dark yellow, oval and imprinted with NVR on one side and ECE on the other. Packs of 7 and 28.
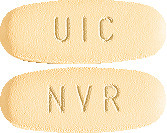
EXFORGE 10/160 (10 mg of amlodipine and 160 mg valsartan) tablets are light yellow, oval and imprinted with NVR on one side and UIC on the other. Packs of 7 and 28.
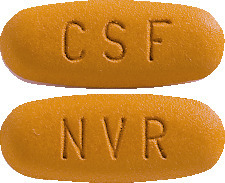
EXFORGE 5/320 (5 mg of amlodipine and 320 mg valsartan) tablets are very dark yellow, oval and imprinted with NVR on one side and CSF on the other. Packs of 7 and 28.
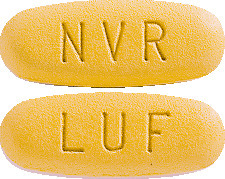
EXFORGE 10/320 (10 mg of amlodipine and 320 mg valsartan) tablets are dark yellow, oval and imprinted with NVR on one side and LUF on the other. Packs of 7 and 28.
Ingredients
EXFORGE tablets contain amlodipine besylate (5 mg or 10 mg) and valsartan (80 mg,160 or 320 mg) as the active ingredient.
The tablets contain the following non active ingredients:
- Cellulose - microcrystalline
- crospovidone
- silica - colloidal anhydrous
- magnesium stearate
- hypromellose
- macrogol 4000
- titanium dioxide
- iron oxide yellow
- talc - purified
EXFORGE 5/320 mg tablets do not contain titanium dioxide.
EXFORGE 5/320 and 10/320 mg tablets also contain sodium starch glycollate.
EXFORGE 10/160 and 5/320 mg tablets also contain iron oxide red.
Sponsor
EXFORGE is supplied in Australia by:
NOVARTIS Pharmaceuticals Australia Pty Limited
ABN 18 004 244 160
54 Waterloo Road
Macquarie Park NSW 2113
Telephone: 1 800 671 203
® = Registered Trademark
This leaflet was prepared in August 2020.
Australian Registration Numbers:
EXFORGE 5/80 (5 mg of amlodipine and 80 mg valsartan) tablets
(AUST R 130787)
EXFORGE 5/160 (5 mg of amlodipine and 160 mg valsartan) tablets
(AUST R 130834)
EXFORGE 10/160 (10 mg of amlodipine and 160 mg valsartan) tablets
(AUST R 130841)
EXFORGE 5/320 (5 mg of amlodipine and 320 mg valsartan) tablets
(AUST R 161820)
EXFORGE 10/320 (10 mg of amlodipine and 320 mg valsartan) tablets
(AUST R 161824)
(Internal reference only: exf260820c.doc based on PI exf260820i.doc)
Published by MIMS October 2020



 The mean incidence of peripheral oedema evenly weighted across all doses was 5.1% with the amlodipine/valsartan combination.
The mean incidence of peripheral oedema evenly weighted across all doses was 5.1% with the amlodipine/valsartan combination.

 Study A2307 was a double blind, placebo controlled, dose response study of 1250 patients with mild to moderate hypertension treated with two combinations of amlodipine and valsartan (10/160, 10/320 mg) or amlodipine alone (10 mg), valsartan alone (160 or 320 mg) or placebo. At week 8 endpoint, the combination treatments were statistically significantly superior to their monotherapy components in reduction of diastolic and systolic blood pressures (see Tables 6 and 7), however, the control rates varied (see Table 8).
Study A2307 was a double blind, placebo controlled, dose response study of 1250 patients with mild to moderate hypertension treated with two combinations of amlodipine and valsartan (10/160, 10/320 mg) or amlodipine alone (10 mg), valsartan alone (160 or 320 mg) or placebo. At week 8 endpoint, the combination treatments were statistically significantly superior to their monotherapy components in reduction of diastolic and systolic blood pressures (see Tables 6 and 7), however, the control rates varied (see Table 8).

 Study A2305 was a double blind, active controlled study of 947 patients with mild to moderate hypertension who were not adequately controlled on valsartan 160 mg. Patients received treatment of two combinations of amlodipine and valsartan (10/160, 5/160 mg) or valsartan alone (160 mg). At week 8 endpoint, the combination treatments were statistically significantly superior to their monotherapy component in reduction of diastolic and systolic blood pressures. (See Table 9.)
Study A2305 was a double blind, active controlled study of 947 patients with mild to moderate hypertension who were not adequately controlled on valsartan 160 mg. Patients received treatment of two combinations of amlodipine and valsartan (10/160, 5/160 mg) or valsartan alone (160 mg). At week 8 endpoint, the combination treatments were statistically significantly superior to their monotherapy component in reduction of diastolic and systolic blood pressures. (See Table 9.) Study A2306 was a double blind, active controlled study of 944 patients with mild to moderate hypertension who were not adequately controlled on amlodipine 10 mg. Patients received a combination of amlodipine and valsartan (10/160 mg) or amlodipine alone (10 mg). At week 8 endpoint, the combination treatment was statistically significantly superior to the monotherapy component in reduction of diastolic and systolic blood pressures. (See Table 10.)
Study A2306 was a double blind, active controlled study of 944 patients with mild to moderate hypertension who were not adequately controlled on amlodipine 10 mg. Patients received a combination of amlodipine and valsartan (10/160 mg) or amlodipine alone (10 mg). At week 8 endpoint, the combination treatment was statistically significantly superior to the monotherapy component in reduction of diastolic and systolic blood pressures. (See Table 10.) Exforge was also studied in an active controlled study of 130 hypertensive patients with diastolic blood pressure ≥ 110 mmHg and < 120 mmHg. In this study (baseline blood pressure 171/113 mmHg), an Exforge regimen of 5/160 mg titrated to 10/160 mg reduced sitting blood pressure at week 6 endpoint by 36/29 mmHg as compared to 32/28 mmHg with a regimen of lisinopril/ hydrochlorothiazide 10/12.5 mg titrated to 20/12.5 mg (not available in Australia).
Exforge was also studied in an active controlled study of 130 hypertensive patients with diastolic blood pressure ≥ 110 mmHg and < 120 mmHg. In this study (baseline blood pressure 171/113 mmHg), an Exforge regimen of 5/160 mg titrated to 10/160 mg reduced sitting blood pressure at week 6 endpoint by 36/29 mmHg as compared to 32/28 mmHg with a regimen of lisinopril/ hydrochlorothiazide 10/12.5 mg titrated to 20/12.5 mg (not available in Australia). (3-ethyl 5-methyl (4RS)-2-[(2-aminoethoxy) methyl]-4-(2-chlorophenyl)- 6-methyl-1,4-dihydropyridine- 3,5-dicarboxylate benzenesulphonate).
(3-ethyl 5-methyl (4RS)-2-[(2-aminoethoxy) methyl]-4-(2-chlorophenyl)- 6-methyl-1,4-dihydropyridine- 3,5-dicarboxylate benzenesulphonate). (N-pentanoyl- N-[2'-(1H-tetrazol-5-yl) biphenyl-4ylmethyl]- L-valine).
(N-pentanoyl- N-[2'-(1H-tetrazol-5-yl) biphenyl-4ylmethyl]- L-valine).