What is in this leaflet
This leaflet answers some common questions about EZOVIR Cold Sore Relief. It does not contain all the available information and does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your pharmacist or doctor have weighed the risks of you taking EZOVIR Cold Sore Relief against the benefits it can provide.
If you have any concerns about taking this medicine, talk to your doctor or pharmacist.
Keep this leaflet with this medicine. You may need to read it again.
What EZOVIR Cold Sore Relief is used for
EZOVIR Cold Sore Relief is an antiviral medicine that is used to treat recurrent outbreaks of cold sores in adults who have a normal immune system (the body system which fights against harmful bacteria, viruses and fungi).
Cold sores are an infection caused by a virus called herpes simplex type I (HSV-1). Cold sores usually begin on or around the lips, mouth, and nose. After redness and swelling develop, the small red bumps turn into fluid-filled blisters.
The blisters may weep or burst, and this can be tender and painful. A shallow ulcer and yellow crust form as the cold sore dries. The crust eventually falls off, exposing new pink-coloured skin. Generally, the sores heal without scarring.
The infection is most commonly acquired as a baby or child from physical contact with parents or relatives that have a cold sore, often by kissing. After the initial infection has healed, the virus becomes dormant in nerve cells.
Cold sores can be unpredictable. Even after many years, some people may experience recurring cold sores due to viral reactivation. Some common triggers to a cold sore may include:
- sun exposure
- stress
- fatigue
- menstrual periods
- fever
- illness
- dry chapped lips
- skin trauma
- a cold
Many people who get cold sores know when one is coming by a tingling, burning, itchy or painful sensation or redness in the area. This can happen very rapidly.
Although EZOVIR Cold Sore Relief does not cure the viral infection, it helps to relieve the symptoms and shorten their duration of an outbreak.
The best results are obtained if the medicine is started as soon as possible after the onset of symptoms occur. These may include tingling, itching or burning, or the appearance of the first signs, such as redness or swelling. This is when the virus is reproducing rapidly.
Ask your pharmacist or doctor if you have any questions about why this medicine has been prescribed for you.
EZOVIR Cold Sore Relief is only available from your pharmacist. The cold sores pack may be purchased without a doctor's prescription. It is not addictive.
Use in Children
This medicine is not recommended for use in infants, children or adolescents under 18 years of age.
Before taking EZOVIR Cold Sore Relief
When you must not take it
Do not take EZOVIR Cold Sore Relief if you have a problem with your body's immune system, which helps fight off infections. Your pharmacist will refer you to your doctor in that case.
Do not take EZOVIR Cold Sore Relief if you have an allergy to:
- famciclovir, the active ingredient
- penciclovir, a related antiviral medicine
- any of the EZOVIR Cold Sore Relief ingredients listed at the end of this leaflet (see 'Product Description')
Some of the symptoms of an allergic reaction may include:
- shortness of breath, wheezing or difficulty breathing;
- swelling of the face, lips, tongue or other parts of the body;
- rash, itching or hives on the skin.
Do not take this medicine if you have a problem with your body's immune system, which helps to fight off infections. Your pharmacist will refer you to your doctor in that case.
Do not take EZOVIR Cold Sore Relief after the expiry date printed on the pack, or if the packaging is torn or shows signs of tampering. In that case, return it to your pharmacist.
Before you start to take it
Tell your pharmacist or doctor if:
- You are over 50 years of age or if you have or have had any medical conditions especially the following:
- A problem with your kidneys.
- Diabetes, high blood pressure, heart problems, liver problems or other medical conditions.
- Signs of an infection other than your cold sore.
Your pharmacist may want to take extra precautions or refer you to a doctor to determine if this medicine is suitable for you.
Tell your doctor if you are pregnant, intend to become pregnant or if you are breastfeeding.
EZOVIR Cold Sore Relief should not be used during pregnancy unless necessary.
Your pharmacist or doctor will discuss with you the potential risks of taking this medicine during pregnancy and will also advise you if you should take this medicine while breast-feeding, based on the benefits and risks of your particular situation.
Tell your doctor or pharmacist if you are prone to allergies or allergic to any other medicines, foods, dyes or preservatives.
If you experience an allergic reaction, stop using the medicine and inform your doctor or pharmacist immediately.
Taking other medicines
Tell your pharmacist or doctor if you are taking any other medicines, including any that you get without a prescription from a pharmacy, supermarket or health food shop.
Some medicines and EZOVIR Cold Sore Relief may interfere with each other. These include:
- probenecid, a prescription medicine used to treat gout (a disease with painful, swollen joints caused by uric acid crystals) and to increase levels of penicillin-type antibiotics
- raloxifene, a medicine used to treat osteoporosis (a disease which causes bones to become less dense, gradually making them weaker, more brittle and likely to break)
- medicines that can affect your immune system
- medicines that can affect your kidneys
You may need to take different amounts of these medicines or may need to take different medicines. Your doctor and pharmacist have more information.
If you have not told your pharmacist or doctor about any of these things, tell him/her before your start taking this medicine.
How to take EZOVIR Cold Sore Relief
Follow all directions given to you by your doctor and pharmacist carefully. These instructions may differ from the information contained in this leaflet.
If you do not understand the instructions on the label, ask your doctor or pharmacist for help.
How much to take
The usual dose is three 500 mg tablets taken together as a single dose.
However, if you have problems with your kidneys and your pharmacist has referred you to your doctor to see if this medicine is suitable for you, your doctor may have recommended a different dose.
Ask your doctor or pharmacist if you are unsure of the correct dose for you. They will tell you exactly how much to take.
Follow the instructions that they give you.
Do not change the dose yourself, without your doctor's advice, regardless of how well you may feel.
When to take it
Take EZOVIR Cold Sore Relief tablets as soon as possible after the first symptoms (e.g. tingling, itching or burning) or signs (e.g. redness or swelling) of a cold sore appear.
Do not take the tablets if a hard crust has already formed on the cold sore.
Keep the tablets for the next episode.
How to take it
Swallow the tablets whole with a full glass of water.
They may be taken with or without food. It is not necessary to chew or crush the tablet.
How long to take it
A single dose of EZOVIR Cold Sore Relief is all that is necessary for treating each episode of cold sores. Each pack of EZOVIR Cold Sore Relief contains enough medicine for one dose. A repeat dose during this episode is not recommended.
If another episode of cold sores recurs, another dose may be taken. However, do not repeat this treatment within 7 days.
If you take too much (Overdose)
Immediately telephone your doctor or the Poisons Information Centre (Australia telephone 13 11 26) for advice, or go to Accident and Emergency at your nearest hospital, if you think you or anyone else may have taken too much EZOVIR Cold Sore Relief. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
Keep the telephone numbers for these places handy.
Taking too much EZOVIR Cold Sore Relief may affect the kidneys. In people who already have kidney problems it may, rarely, lead to kidney failure if their dose is not correctly lowered.
While you are taking EZOVIR Cold Sore Relief
Things you must do
Tell your pharmacist or doctor if your cold sore symptoms do not improve within a few days, or if they become worse.
If you become pregnant, tell your doctor before taking any further doses of this medicine to another episode of cold sores. Your doctor can discuss with you the risks of taking it while you are pregnant.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you periodically take this medicine to treat recurring episodes of cold sores.
Tell any other doctor, dentist or pharmacist who treats you that you are taking EZOVIR Cold Sore Relief.
Things you must not do
Do not take less than the recommended dose of 3 tablets, unless advised by your doctor.
Do not give this medicine to anyone else even if their condition seems to be the same as yours.
Do not use this medicine to treat any other complaints unless your doctor tells you to.
Things to be careful of
If you are pregnant or breastfeeding, ask your doctor or pharmacist for advice before taking any medicine.
Be careful driving, operating machinery or doing jobs that require you to be alert until you know how EZOVIR Cold Sore Relief affects you.
This medicine can cause dizziness, sleepiness or confusion in some people.
Things that may help your condition
Cold sores are contagious, and the virus can be passed on from an infected person to an uninfected person through close physical contact or saliva, even when blisters are not present.
The risk is much higher when the cold sore is visible, as the virus can be shed, making it easy to infect other people.
Take the following precautions to avoid spreading the virus:
- Keep the areas affected by the virus as clean and dry as possible.
- Avoid touching or scratching the sore areas as you may spread the virus on your fingers.
- Do not share any objects that have been in contact with a cold sore (e.g. drinking glasses, eating utensils, or towels).
- Avoid direct skin-to-skin contact of the area with other people (e.g. kissing) until the cold sore has healed.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking EZOVIR Cold Sore Relief.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Do not be alarmed by the below lists of possible side effects. You may not experience any of them. Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you notice any of the following and they worry you:
- headache
- dizziness
- nausea (feeling sick) or vomiting
- diarrhoea
- itching or an itchy rash (urticaria)
The above side effects are usually mild.
Tell your doctor as soon as possible if you notice any of the following:
- a rash elsewhere on the body, that is separate from the cold sore
- extreme sleepiness or confusion, usually in older people
- hallucinations (seeing or hearing things that are not really there)
- painful or swollen joints
- aching muscles or muscle tenderness or weakness that is not caused by exercise
- yellowing of the skin or eyes (signs of jaundice)
- palpitations (signs of abnormal heart beat)
The above side effects may need medical attention.
Tell your doctor immediately or go to Accident and Emergency at your nearest hospital if any of the following side effects happen:
- swelling below the surface of the skin (e.g. swelling around the face, eye, eyelid or throat)
- unexplained bruising or purplish patches on the skin or bleeding more easily than usual, as it may indicate that the number of platelets (a type of blood cell responsible for blood clotting) in your blood are reduced
- severe blistering of the skin or mucous membranes of the lips, eyes, mouth, nasal passages or genitals (signs of a serious skin reaction)
- purple patches, itching, burning of the skin (signs of inflamed blood vessels)
- seizures or fits
- difficulty breathing or swallowing, wheezing or cough, light-headedness, changes in alertness, skin reddening, facial/throat swelling, blue discolouration of the lips, tongue or skin (signs of severe allergic reaction)
- signs of a possible liver problem such as persistent pain in the upper right abdomen, yellowing of the skin and/or eyes, dark urine or pale bowel motions
The above side effects are very rare.
Tell your doctor if you notice anything else that is making you feel unwell. Other side effects not listed here or not yet known may happen in some people.
Ask your doctor or pharmacist to answer any questions you may have.
After taking EZOVIR Cold Sore Relief
Storage
Keep your medicine in the original container until it is time to take it. Store your EZOVIR Cold Sore Relief tablets in a dry place where the temperature stays below 25°C.
Do not leave the tablets in the car or on window sills. Heat and dampness can destroy some medicines. EZOVIR Cold Sore Relief tablets will keep best if they are stored cool and dry.
Keep the medicine where children cannot reach it. A locked cupboard at least one-and-a-half meters above the ground is a good place to store medicines.
Disposal
If your pharmacist or doctor recommended that you take less than the full dose in this pack, told you to stop taking your medicine or if it has expired, ask your pharmacist what to do with any tablets that you may have left over.
Product description
What it looks like
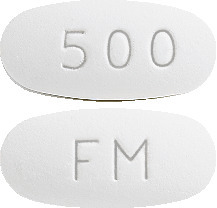
EZOVIR Cold Sore Relief 500 mg tablets are are white, oval, film-coated tablets with "FM" on one side and "500" on the other. Each carton contains 3 tablets.
Ingredients
EZOVIR Cold Sore Relief tablets contains 500 mg of famciclovir as the active ingredient.
The tablets also contain the following inactive ingredients:
- microcrystalline cellulose
- crospovidone
- silicon dioxide
- copovidone
- sodium stearylfumarate
- Opadry II complete film coating system YS-22-18096 White
Supplier
EZOVIR Cold Sore Relief is supplied in Australia by:
Alphapharm Pty Ltd trading as Viatris
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.viatris.com.au
Phone: 1800 274 276
This leaflet was prepared on
March 2023.
Australian registration numbers:
EZOVIR Cold Sore Relief famciclovir 500 mg tablets
- AUST R 351702
EZOVIR COLD SORE RELIEF_cmi\Mar23/00
Published by MIMS May 2023



 Plasma concentration-time curves of penciclovir are similar following single and repeat (b.i.d. and t.i.d.) dosing and there is no accumulation of penciclovir on repeated dosing. Penciclovir and its 6-deoxy precursor are poorly (< 20%) bound to plasma proteins. Famciclovir is eliminated principally as penciclovir and its 6-deoxy precursor, which are excreted in urine. No unchanged famciclovir has been detected in urine. Tubular secretion and glomerular filtration contribute to renal elimination of the compound. The terminal plasma half-life of penciclovir after both single and repeat dosing with famciclovir is approximately 2.0 hours. There is no accumulation of penciclovir on repeated dosing with famciclovir.
Plasma concentration-time curves of penciclovir are similar following single and repeat (b.i.d. and t.i.d.) dosing and there is no accumulation of penciclovir on repeated dosing. Penciclovir and its 6-deoxy precursor are poorly (< 20%) bound to plasma proteins. Famciclovir is eliminated principally as penciclovir and its 6-deoxy precursor, which are excreted in urine. No unchanged famciclovir has been detected in urine. Tubular secretion and glomerular filtration contribute to renal elimination of the compound. The terminal plasma half-life of penciclovir after both single and repeat dosing with famciclovir is approximately 2.0 hours. There is no accumulation of penciclovir on repeated dosing with famciclovir. Chemical name: 9-(4-acetoxy-3-acetoxymethylbut-1-yl)-2-aminopurine.
Chemical name: 9-(4-acetoxy-3-acetoxymethylbut-1-yl)-2-aminopurine.