What is in this leaflet
This leaflet answers some common questions about Fenocol.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor or pharmacist has weighed the risks of you taking this medicine against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What Fenocol is used for
Fenocol is used to help regulate cholesterol and triglycerides which are fat-like substances in the blood.
Cholesterol is present in many foods and is also made in your body by the liver. If your body does not balance the amount of cholesterol it needs with the amount of cholesterol eaten, then your cholesterol becomes too high.
High cholesterol is more likely to occur with certain diseases or if you have a family history of high cholesterol.
When you have high levels of cholesterol it may 'stick' to the inside of your blood vessels instead of being carried to the parts of the body where it is needed.
Over time, this can form hard areas (called plaque) on the walls of your blood vessels, making it more difficult for the blood to flow. This blocking of your blood vessels can lead to heart disease (such as heart attack and angina), and stroke.
Cholesterol is carried through the body by different proteins, LDL and HDL. LDL cholesterol is the 'bad' cholesterol that can block your blood vessels. HDL cholesterol is the 'good' cholesterol that is thought to remove the 'bad' cholesterol from the blood vessels.
In most patients, Fenocol reduces the bad cholesterol and can actually raise the good cholesterol.
Fenocol does not reduce the cholesterol that comes from fat in food.
Therefore, when you are taking Fenocol, you also need to follow a low-fat diet and other measures, such as exercise and weight control.
How Fenocol works
Fenocol works through the activation of a cell nuclear receptor called PPARα, which reduces the amount of triglycerides and bad cholesterol made in the body and increases the good cholesterol.
Fenocol belongs to a group of medicines known as fibric acid derivatives.
Ask your doctor if you have any questions about why it has been prescribed for you. Your doctor may have prescribed it for another purpose.
This medicine is not addictive.
This medicine is available only with a doctor's prescription.
Before you take Fenocol
When you must not take it
Do not take Fenocol if you have an allergy to:
- any medicine containing fenofibrate
- any fibrates (such as gemfibrozil)
- ketoprofen
- any of the ingredients listed at the end of this leaflet
Some symptoms of an allergic reaction include skin rash, itching, shortness of breath or swelling of the face, lips or tongue, which may cause difficulty in swallowing or breathing.
Do not take this medicine if you are pregnant. It may affect your developing baby if you take it during pregnancy.
Do not breastfeed if you are taking this medicine. The active ingredient in Fenocol passes into breast milk and there is a possibility your baby may be affected.
Do not take this medicine if you have:
- liver disease
- severe kidney disease
- disease of the gallbladder or pancreas
- experienced muscle pain, tenderness or weakness from other medicines used to treat high cholesterol or triglycerides.
Do not take this medicine if you are taking another fibrate.
Do not take this medicine if you are allergic to peanuts, peanut oil, soy lecithin or related products.
Do not give Fenocol to anyone under the age of 18 years. The safety and effectiveness of Fenocol in children has not been established.
Do not take it after the expiry date printed on the pack or if the packaging is damaged or shows signs of tampering. If it has expired or is damaged return it to your pharmacist for disposal.
Before you start to take it
Tell your doctor if you have any allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor or pharmacist if you have or have had any of the following medical conditions:
- kidney problems
- muscular aching, tenderness or weakness not caused by exercise
Tell your doctor if you are pregnant, intend to become pregnant, or are breastfeeding. Your doctor can discuss the risks and benefits involved.
If you have not told your doctor about any of the above, tell them before you take Fenocol.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you buy without a prescription from your pharmacy, supermarket or health food store.
Some medicines may interfere with each other. These include:
- oral anti-coagulants (medicines used to prevent blood clots)
- other cholesterol regulating medicines including fibrates
- ciclosporin (a medicine which suppresses the immune system)
- glitazones (medicines to reduce sugar levels)
These medicines may be affected by Fenocol or may affect how well it works. You may need to use different amounts of your medicine or take different medicines.
Your doctor has more information on medicines to be careful with or to avoid while taking Fenocol.
How to take Fenocol
Note: A lower strength fenofibrate 48 mg tablets can be available from other brand/s.
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the box, ask your doctor or pharmacist for help.
How much to take
The initial recommended dose is 145 mg daily, taken as 1 x 145 mg tablet.
How to take it
Swallow the tablet(s) whole with a full glass of water.
When to take it
Fenocol can be taken at any time of the day, with or without food. Any dietary measures started before treatment with Fenocol should be continued.
Take Fenocol at about the same time each day. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
How long to take it
Continue taking your medicine for as long as your doctor or pharmacist tells you.
Fenocol helps to regulate your levels of cholesterol (both LDL and HDL) and triglycerides. It does not cure your condition. Therefore, you must continue to take it as directed by your doctor if you expect to keep your levels controlled. If you stop taking Fenocol, your levels may become abnormal again.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember, and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for the dose that you missed. This may increase the chance of getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering when to take your medicine, ask your pharmacist for advice.
If you take too much (overdose)
Immediately telephone your doctor, or the Poisons Information Centre (telephone Australia 13 11 26) or go to Accident and Emergency at your nearest hospital if you think you or anyone else may have taken too much Fenocol. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
Symptoms of an overdose may include:
- diarrhoea
- nausea
While you are using Fenocol
Things you must do
Have your blood fats checked when requested by your doctor to make sure Fenocol is working.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking Fenocol.
Tell any other doctors, dentists and pharmacists who treat you that you are taking this medicine.
If you are going to have surgery, tell the surgeon that you are taking this medicine.
If you become pregnant while you are taking this medicine, tell your doctor or pharmacist immediately.
Things you must not do
Do not use this medicine to treat any other complaints unless your doctor or pharmacist tells you to.
Do not give this medicine to anyone else, even if they have the same condition as you.
Do not stop taking Fenocol, or change the dosage, without checking with your doctor.
Things to be careful of
Be careful driving or operating machinery until you know how Fenocol affects you. This medicine may cause dizziness in some people.
Avoid drinking large quantities of alcohol while you are taking this medicine. Drinking large quantities of alcohol may increase your chance of Fenocol causing liver problems.
Things that may help you reduce the chance of coronary heart disease
Lowering high cholesterol can help reduce your chances of having Coronary Heart Disease (CHD). However, your chances of having CHD may be increased by several other factors including high blood pressure, cigarette smoking, diabetes, excess weight, family history of CHD, being a male and being a woman who has reached menopause.
Some self-help measures suggested below may help your condition and help reduce your chances of having CHD. Talk to your doctor, pharmacist, or dietician about these measures and for more information.
- Diet - continue the low-fat diet recommended by your doctor, dietician or pharmacist
- Weight - your doctor may advice you to lose weight if you are overweight
- Exercise - make exercise a part of your routine-walking is good. Ask your doctor for advice before starting exercise.
- Smoking - your doctor will advise you to stop smoking
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Fenocol.
It helps most people with high cholesterol, but it may have unwanted side effects in a few people.
All medicines have some unwanted side effects.
Sometimes they are serious, but most of the time they are not. You may need medical attention if you get some of the side-effects.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- stomach pain or discomfort
- back pain
- headache
- muscular pain or spasms
- unusual tiredness or weakness
- diarrhoea or constipation
- nausea
- skin reactions, photosensitivity reactions
- sexual dysfunction
The above list includes the more common side effects of your medicine. They are usually mild and short-lived.
If any of the following happen, tell your doctor immediately or go to Accident and Emergency at your nearest hospital:
- difficulty in breathing
- severe abdominal pain
- chest pain
- temporary paralysis of the muscle
- yellowing of the skin and eyes and dark coloured urine
The above list includes very serious side effects. You may need urgent medical attention. These side effects are rare.
Tell your doctor or pharmacist if you notice anything else that is making you feel unwell. Other side effects not listed above may occur in some people.
After using Fenocol
Storage
Keep your tablets in the pack until it is time to take them. If you take the tablets out of the box or the blister pack, they may not keep well.
Keep the medicine in a cool, dry place where the temperature stays below 30°C.
Do not store it or any other medicine in the bathroom, near a sink, or on a windowsill. Do not leave it in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a- half metres above the ground is a good place to store medicines.
Disposal
If your doctor or pharmacist tells you to stop taking this medicine, or the medicine has passed its expiry date, ask your pharmacist what to do with any that are left over.
Product description
What it looks like
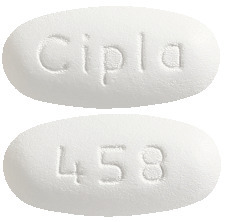
Fenocol 145 mg film-coated tablets are white to off white coloured, oval shaped, biconvex film coated tablet, debossed with 'cipla' on one side and code '458' on other side.
The 145 mg strength is available in boxes of 10 and 30 tablets.
Ingredients
Fenocol contains 145 mg of fenofibrate as the active ingredient.
The tablets also contain the following inactive ingredients:
Tablets core:
- sucrose,
- hypromellose,
- sodium lauryl sulfate,
- lactose monohydrate,
- silicified microcrystalline cellulose,
- crospovidone,
- docusate sodium,
- magnesium stearate.
Contains sugars as lactose.
Tablets coating
OPADRY AMB-White OY-B-28920 (PI no. 10274):
- Polyvinyl alcohol-part hydrolyzed,
- titanium dioxide,
- talc,
- lecithin (soya),
- xanthan gum.
Supplier
Arrow Pharma Pty Ltd
15-17 Chapel Street
Cremorne VIC 3121
Australian Registration Number
AUST R 288060
This leaflet was prepared in November 2021.
Published by MIMS January 2022