What is in this leaflet
This leaflet answers some common questions about FIBSOL.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking FIBSOL against the benefits they expect it will have for you.
Talk to your doctor or pharmacist if you have any concerns about taking this medicine.
Keep this leaflet with your medicine. You may need to read it again.
What FIBSOL is used for
FIBSOL is used to treat:
- high blood pressure (hypertension)
- heart failure
- patients who have had a heart attack.
FIBSOL belongs to a group of medicines called angiotensin converting enzyme (ACE) inhibitors.
How FIBSOL works
FIBSOL works by widening your blood vessels, making it easier for your heart to pump blood around your body.
This helps increase the supply of oxygen to your heart, so that when you place extra demands on your heart, such as during exercise, your heart may cope better and you may not get short of breath as easily. By increasing the supply of oxygen to your heart, your heart does not have to work as hard which may reduce the risk of further damage to your heart after you have a heart attack.
Your doctor may have prescribed FIBSOL for another reason. Ask your doctor if you have any questions about why FIBSOL has been prescribed for you.
FIBSOL is not addictive.
This medicine is available only with a doctor's prescription.
Use in children
The safety and effectiveness of FIBSOL in children have not been established.
Before you take it
When you must not take it
Do not take FIBSOL if:
- you are allergic to FIBSOL or any other medicine containing lisinopril, or any of the ingredients listed at the end of this leaflet.
Symptoms of an allergic reaction to FIBSOL may include skin rash, itchiness, shortness of breath, swelling of the face, lips or tongue, muscle pain or tenderness or joint pain. - you have taken any other “ACE inhibitor” medicine before, which caused your face, lips, tongue, throat, hands or feet to swell up, or made it hard for you to breath.
If you have had an allergic reaction to an ACE inhibitor before, you may be allergic to FIBSOL. - you have a history of swelling of the face, lips, tongue, throat, hands or feet, for no apparent reason.
Do not take FIBSOL if you are pregnant. It may affect your developing baby if you take it during pregnancy.
Do not take FIBSOL if you are breastfeeding. This medicine passes into breast milk and therefore there is a possibility that the breast-fed baby may be affected.
Do not take FIBSOL if the expiry date (EXP) printed on the pack has passed.
Do not take this medicine if the packaging is torn or shows signs of tampering or deterioration. If it has expired, damaged or shows any signs of deterioration (e.g. darkening of the tablets), return it to your pharmacist.
Do not take FIBSOL if you are undergoing haemodialysis.
Do not take FIBSOL if you are taking blood pressure medicine containing aliskiren, and you have diabetes mellitus or kidney problems.
If you are not sure whether you should start taking FIBSOL, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to:
- any other medicines
- any other substances, such as foods, preservatives or dyes.
Tell your doctor if you have or have had any medical conditions, especially the following:
- kidney problems, or are having dialysis
- liver problems
- heart problems
- low blood pressure, which you may notice as dizziness or lightheadedness
- diabetes
- aortic stenosis (narrowing of the aorta), renal stenosis (narrowing of the renal artery) or hypertrophic cardiomyopathy (an increase in the thickness of the heart muscle)
- diarrhoea or vomiting
- high levels of potassium in your blood.
Tell your doctor if you are following a very low salt diet.
Tell your doctor if you are about to receive desensitisation therapy for an allergy.
Tell your doctor if you are scheduled to have surgery under general anaesthetic. Your blood pressure may drop.
Tell your doctor if you are taking medicines called mTOR inhibitors (such as temsirolimus, everolimus, sirolimus) or medicines containing NEP inhibitors (such as racecadotril). Taking these medicines with FIBSOL may increase the risk of angioedema. Signs of angioedema include swelling of the face, lips, tongue and/or throat with difficulty in swallowing of breathing.
Tell your doctor if you plan to become pregnant or breastfeed.
If you have not told your doctor about any of the above, tell them before you start taking FIBSOL.
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and FIBSOL may interfere with each other. These include:
- other medicines used to treat high blood pressure or heart problems called angiotensin II receptor blockers, such as candesartan, valdesartan, telmisartan, olmesartan or irbesartan
- other medicines used to treat high blood pressure, including any that contain aliskiren
- diuretics, also known as fluid or water tablets
- non steroidal anti-inflammatory drugs (NSAIDs), medicines used to relieve pain, swelling and other symptoms of inflammation, including arthritis
- potassium supplements or potassium-containing salt substitutes
- lithium, a medicine used to treat mood swings and some types of depression
- heparin, a medicine used to treat blood clots
- gold injections (such a sodium aurothiomalate) usually used to treat rheumatoid arthritis
- insulin or other medicines used to treat diabetes
- medicines called mTOR inhibitors (such as temsirolimus, everolimus, sirolimus) or medicines containing NEP inhibitors (such as racecadotril)
These medicines may be affected by FIBSOL, or may affect how well it works. You may need different amounts of your medicine, or you may need to take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking FIBSOL.
How to take FIBSOL
Follow all directions given to you by your doctor and pharmacist carefully. They may differ from the information contained in this leaflet.
Your doctor or pharmacist will tell you how many tablets you will need to take each day and when to take them. This depends on your condition and whether or not you are taking any other medicines.
If you do not understand the instructions on the container, ask your doctor or pharmacist.
How much to take
FIBSOL is available as 5 mg, 10 mg and 20 mg tablets and is taken as a single daily dose.
The dose that your doctor prescribes will depend upon your condition.
High blood pressure (hypertension)
The usual recommended starting dose is 5 mg to 10 mg taken once a day.
The usual maintenance dose is 10 mg to 20 mg taken once a day.
Heart failure
The usual starting dose is 2.5 mg taken once a day.
The usual effective dosage is 5 mg to 20 mg taken once a day.
Heart attack
FIBSOL may be started within 24 hours of the onset of the symptoms of heart attack. The starting dose is 5 mg, followed by 5 mg after 24 hours, 10 mg after 48 hours and then 10 mg taken once a day thereafter.
How to take it
Swallow FIBSOL whole with a full glass of water.
When to take it
Take FIBSOL at about the same time each day. Taking your tablets at about the same time each day will have the best effect. It will also help you remember when to take the tablets.
It does not matter if you take FIBSOL before or after food.
How long to take it
FIBSOL helps control your condition, but does not cure it. Therefore you must take FIBSOL every day. Continue taking your medicine for as long as your doctor tells you.
If you forget to take FIBSOL
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember, and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for the dose you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your tablets, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor, or the Poisons Information Centre (telephone 13 11 26), or go to Accident and Emergency at the nearest hospital, if you think you or anyone else may have taken too much FIBSOL. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
If you take too much FIBSOL, you may feel lightheaded, dizzy or faint.
While you are taking it
Things you must do
Tell any other doctors, dentists and pharmacists who are treating you that you are taking FIBSOL.
If you are about to be started on any new medicine, tell your doctor, dentist or pharmacist that you are taking FIBSOL.
Make sure you drink enough water during exercise and hot weather when you are taking FIBSOL, especially if you sweat a lot.
If you do not drink enough water while taking this medicine, you may faint or feel light-headed or sick. This is because your blood pressure is dropping suddenly. If you continue to feel unwell, tell your doctor.
If you have excessive vomiting or diarrhoea while taking FIBSOL tell your doctor. You may lose too much water and salt and your blood pressure may drop too much.
If you feel light-headed or dizzy after taking your first dose of FIBSOL, or when your dose is increased, tell your doctor immediately. This is especially important if you are taking this medicine for heart failure.
If you plan to have surgery that needs a general anaesthetic, tell your doctor or dentist that you are taking FIBSOL. Your blood pressure may drop suddenly.
If you become pregnant while taking FIBSOL, tell your doctor immediately.
Tell your doctor that you are taking FIBSOL if you are about to have any blood tests. It may interfere with the results of some tests.
Have your blood pressure checked when instructed by your doctor, to make sure FIBSOL is working.
Go to your doctor regularly for a check-up. Your doctor may occasionally do a blood test to check your potassium levels and see how your kidneys are working.
Things you must not do
Do not give FIBSOL to anyone else, even if they have the same condition as you.
Do not take FIBSOL to treat any other complaints unless your doctor or pharmacist tells you to.
Do not stop taking FIBSOL, or lower the dosage, without checking with your doctor.
Things to be careful of
If you feel lightheaded, dizzy or faint when getting out of bed or standing up, get up slowly. Standing up slowly, especially when you get up out of bed or chairs, will help your body get used to the change in position and blood pressure. If this problem continues or gets worse, talk to your doctor.
Be careful driving or operating machinery until you know how FIBSOL affects you.
As with other ACE inhibitors, FIBSOL may cause dizziness, lightheadedness, tiredness and drowsiness in some people. Make sure you know how you react to FIBSOL before you drive, operate machinery or do anything else that could be dangerous if you are dizzy or lightheaded. If this occurs do not drive. If you drink alcohol, dizziness or lightheadedness may be worse.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking FIBSOL.
FIBSOL helps most people with hypertension, heart failure and people who have had a heart attack, but it may have unwanted side effects in a few people. All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Ask your doctor or pharmacist to answer any questions you may have.
Following is a list of possible side effects. Do not be alarmed by this list. You may not experience any of them.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- light headedness or dizziness
- headache
- fatigue
- dry cough
- mild stomach upsets such as feeling sick, diarrhoea or stomach pain
- hair loss or thinning
- impotence
- changes in the way things taste
- feeling sleepy or difficulty in going to sleep, strange dreams
- runny nose or sinus pain
- changes in the way things smell.
These side effects are usually mild but may be serious.
Tell your doctor as soon as possible if you notice any of the following:
- fast or irregular heart beats
- fainting
- yellowing of the skin and/or eyes
- itchy skin rash or other skin problems
- signs of frequent or worrying infections such as fever, mouth or tongue ulcers
- tingling or numbness of the hands and feet
- bruising more easily than normal
- severe abdominal pain
- unusual tiredness or weakness, fatigue
- passing less urine than is normal for you
- swelling of the hands, feet or ankles
- any severe skin reaction
- signs of dehydration such as nausea, vomiting, muscle cramps, headache, drowsiness and tiredness
- seeing, feeling or hearing things that are not there.
These may be serious side effects. You may need medical attention. Serious side effects are rare.
If any of the following happen, stop taking FIBSOL and either tell your doctor immediately or go to Accident and Emergency at the nearest hospital:
- swelling of the face, lips, mouth or throat which may cause difficulty in swallowing or breathing
- pink or red itchy spots on the skin which may blister and progress to form raised, red, pale-centred marks
- chest pain or feeling of tightness, pressure or heaviness in the chest
- collapse, numbness or weakness in the arms or legs
- severe flaking or peeling of the skin.
These are very serious side effects. You may need urgent medical attention or hospitalisation. These side effects are very rare.
Other side effects not listed above may also occur in some patients. Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
After using it
Storage
Keep your tablets in the pack until it is time to take them. If you take the tablets out of the pack they may not keep well.
Keep your tablets in a cool dry place where the temperature stays below 25°C.
Do not store FIBSOL or any other medicine in the bathroom or near a sink.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor or pharmacist tells you to stop taking FIBSOL or the tablets have passed their expiry date, ask your pharmacist what to do with any that are left over.
Product description
What it looks like
FIBSOL 5 mg tablets
Off white round tablet, debossed with “L” break-line “5” on one side and plain on the other side.
FIBSOL 10 mg tablets
Off white round tablet, debossed with “L” break-line “10” on one side and plain on the other side.
FIBSOL 20 mg tablets
Off white round tablet, debossed with “L” break-line “20” on one side and plain on the other side.
FIBSOL is available in blister packs of 30 tablets.
Ingredients
Active ingredient:
- lisinopril (as dihydrate).
Other ingredients:
- mannitol
- calcium hydrogen phosphate
- maize starch
- pregelatinised maize starch
- colloidal anhydrous silica
- magnesium stearate.
FIBSOL does not contain lactose, sucrose, gluten, tartrazine or any other azo dyes.
Sponsor
Arrotex Pharmaceuticals Pty Ltd
15 – 17 Chapel Street
Cremorne VIC 3121
Australian registration numbers
5 mg Aust R 101316
10 mg Aust R 101317
20 mg Aust R 101318
This leaflet was revised in August 2023.
Published by MIMS October 2023


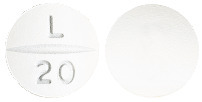

 Molecular formula: C21H31N3O5.2H2O. Molecular weight: 441.53.
Molecular formula: C21H31N3O5.2H2O. Molecular weight: 441.53.