What is in this leaflet
This leaflet answers some common questions about GAPENTIN.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking GAPENTIN against the benefits they expect it will have for you.
Ask your doctor or pharmacist if you have any concerns about taking this medicine.
Keep this leaflet with the medicine. You may need to read it again.
What GAPENTIN is used for
GAPENTIN capsules contain gabapentin as the active ingredient.
GAPENTIN belongs to a group of medicines called anticonvulsants and is used to control epilepsy. It is also used in addition to current anti-epileptic therapy when the current treatment is no longer working as well. Epilepsy is a condition where you have repeated seizures (fits). There are many different types of seizures, ranging from mild to severe.
This medicine is also used to treat neuropathic pain, a type of pain caused by damage to the nerves
How Gapentin works
Anticonvulsants such as GAPENTIN are thought to work by controlling brain chemicals which send signals to nerves so that seizures are treated.
Gapentin also has pain relieving effects
Your doctor may have prescribed Gapentin in addition to other medicines that you may be taking. This may be necessary if your current treatment is no longer working as well.
Ask your doctor if you have any questions about why GAPENTIN has been prescribed for you. Your doctor may have prescribed it for another reason.
Gapentin may lead to dependence on this medicine.
This medicine is available only with a doctor’s prescription.
Use in children
It is not recommended for use in children under the age of 3 years to control epilepsy, as its safety and effectiveness in that age group have not been established. Also, the safety and effectiveness of GAPENTIN for the treatment of neuropathic pain in children under the age of 18 years have not been established.
Before you take it
When you must not take it
Do not take GAPENTIN if you have an allergy to:
- gabapentin
- any of the ingredients listed at the end of this leaflet.
Symptoms of an allergic reaction may include shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue or other parts of the body; rash, itching or hives on the skin.
Do not take it after the expiry date (EXP) printed on the pack. Do not take it if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
Talk to your doctor or pharmacist if you are not sure whether you should start taking GAPENTIN.
If you are not sure whether you should start taking GAPENTIN, talk to your doctor or pharmacist.
Before you start to take it
Tell your doctor or pharmacist if you have allergies to:
- any other medicines, especially barbiturates or any other anticonvulsant medicines
- any other substances, such as foods, preservatives or dyes.
Tell your doctor if you have or have had any medical conditions, especially the following:
- kidney problems
- mixed seizure disorders that include absence seizures.
Tell your doctor if you have a history of drug abuse and/ or psychiatric disorders. Gapentin poses risks of abuse and dependence. Your body may become used to you taking Gapentin and this may result in physical dependence. It means that you may experience withdrawal symptoms if you stop taking Gapentin suddenly. suddenly. So it is important to strictly follow the directions given by your doctor.
Tell your doctor if you are pregnant or intend to become pregnant. This medicine may affect your developing baby if you take it during pregnancy. However, it is very important to control your fits while you are pregnant. If it is necessary for you to take GAPENTIN, your doctor can help you decide whether or not to take it during pregnancy.
Tell your doctor if you are breast-feeding or plan to breastfeed. Your doctor will discuss the possible risks and benefits of using this medicine during breastfeeding. GAPENTIN passes into breast milk. The effect on your breast-fed baby is unknown.
If you do breastfeed, watch your baby carefully. If your baby develops a skin rash, becomes sleepy or has unusual symptoms, don’t breastfeed again until you speak to your doctor.
Your doctor can discuss the risks and benefits of breast-feeding with you.
If you have not told your doctor about any of the above, tell them before you start taking GAPENTIN.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including all prescription medicines and all medicines, vitamins, herbal supplements or natural therapies you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and GAPENTIN may interfere with each other. These include:
- some medicines used to treat stomach or duodenal ulcers, such as cimetidine
- antacids, medicines used to treat heartburn or reflux
- opioids, medicines used to treat severe pain e.g. morphine.
Your doctor and pharmacist may have more information on medicines to be careful with or avoid while taking GAPENTIN.
These medicines may be affected by GAPENTIN or may affect how well it works. You may need different amounts of your medicine or you may need to take different medicines. Your doctor or pharmacist will advise you.
Gapentin and certain other medicines may influence each other.
Using Gapentin with other medicines that can make you feel drowsy, such as sleeping tablets and other pain relievers (e.g. benzodiazepines and opioids), antihistamines, antidepressants, antipsychotics, cannabis, and alcohol may result in severe drowsiness, decreased awareness, breathing problems, coma and death. Your doctor will minimise the dose and duration of use; and monitor you for signs and symptoms of breathing difficulties and sedation.
How to take it
How much to take
Your doctor will tell you how many capsules you will need to take each day. This may depend on your age, your condition and whether or not you are taking any other medicines.
Your doctor may recommend that you start with a low dose and slowly increase the dose to the lowest amount needed to control your epilepsy/convulsions or neuropathic pain.
Follow all directions given to you by your doctor carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the pack, ask your doctor or pharmacist for help.
How to take it
Swallow GAPENTIN whole with a full glass of water.
When to take it
Take your medicine at about the same time each day. Taking GAPENTIN at the same time each day will have the best effect. It will also help you remember when to take the capsules.
If you are taking Gapentin three times a day, do not allow more than 12 hours between doses.
It does not matter if you take it before or after food.
If you forget to take it
If you have missed a dose by not more than 4 hours, take the dose as soon as you remember.
However, if you have missed a dose by more than 4 hours, you should skip the dose and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for the dose that you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take Gapentin, ask your pharmacist for help.
How long to take it
Continue taking GAPENTIN for as long as your doctor tells you to.
This medicine helps control your condition, but does not cure it. Therefore you must take your medicine every day, even if you feel well.
Do not stop taking GAPENTIN or lower the dosage, without checking with your doctor.
Do not let yourself run out of medicine over the weekend or on holidays.
Stopping GAPENTIN suddenly may worsen your condition or increase your chance of experiencing withdrawal symptoms, such as sleeplessness, headache, nausea (feeling sick), anxiety, excessive sweating or diarrhoea (runny stools). If appropriate your doctor will slowly reduce your dose before you can stop taking it completely.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at your nearest hospital, if you think that you or anyone else may have taken too much GAPENTIN. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
Symptoms of overdose include may include feeling drowsy, weak, unsteady when walking, double vision, slurred speech or diarrhoea.
While you are taking it
Things you must do
Tell any other doctors, dentists, and pharmacists who are treating you that you are taking GAPENTIN.
If you are about to be started on any new medicine or before you have any surgery or emergency treatment, tell any other doctor, dentist or pharmacist that you are taking this medicine.
Tell your doctor immediately if you have any thoughts of suicide or self-harm, any unusual changes in mood or behaviour, or show signs of depression. Some people being treated with antiepileptics, such as GAPENTIN, have had thoughts of harming or killing themselves.
Patients and caregivers should be alert and monitor for signs and symptoms of suicide, these include:
- thoughts or talk of death or suicide
- thoughts or talk of self-harm or harm to others
- any recent attempts of self-harm
- new or an increase in aggressive behaviour, irritability or agitation
- new onset of or worsening of depression.
Mention of suicide or violence must be taken seriously.
If you or someone you know is demonstrating these warning signs and symptoms of suicide while taking GAPENTIN, contact your doctor or a mental health professional right away.
Tell your doctor if you feel GAPENTIN is not helping your condition. Your doctor may need to change your medicine.
Tell your doctor if, for any reason, you have not taken GAPENTIN exactly as prescribed. Otherwise, your doctor may change your treatment unnecessarily.
Tell your doctor if you become pregnant while taking this medicine.
Tell your doctor if you need to have any medical tests while you are taking it. GAPENTIN may affect the results of some tests.
If you are going to have any surgery or procedure, including dental surgery, tell your surgeon, doctor or dentist that you are taking this medicine.
Be sure to keep all of your doctor’s appointments so that your progress can be checked. Your doctor will check your progress and may want to take some tests from time to time. This helps to prevent unwanted side effects.
Things you must not do
Do not give GAPENTIN to anyone else, even if their symptoms seem similar to yours or they have the same condition as you.
Do not take it to treat any other complaints unless your doctor tells you to.
Do not stop taking your medicine unless your doctor tells you to. Stopping GAPENTIN suddenly, if you have epilepsy, may cause unwanted side effects or make your condition worse. Your doctor will slowly reduce your dose before you can stop taking it completely.
Things to be careful of
Be careful driving or operating machinery until you know how GAPENTIN affects you.
As with other anticonvulsant medicines, GAPENTIN may cause drowsiness, dizziness, lightheadedness or sleepiness in some people.
Make sure you know how you react to it before you drive a car, operate machinery, or do anything else that could be dangerous if you are dizzy or light-headed. If this occurs do not drive.
Children should not ride a bike, climb trees or do anything else that could be dangerous if they are feeling drowsy or sleepy.
Be careful when drinking alcohol while taking it. If you drink alcohol, dizziness or light-headedness, sleepy may be worse. Your doctor may suggest you avoid alcohol while you are being treated with GAPENTIN.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking GAPENTIN.
This medicine helps most people with epilepsy or neuropathic pain, but it may have unwanted side effects in a few people. All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
It can be difficult to tell whether side effects are the result of taking Gapentin, of your condition, or side effects of the other medicines you may be taking, for this reason it is important to tell your doctor of any change in your condition.
If you are over 65 years of age you may have an increased chance of getting side effects.
Ask your doctor or pharmacist to answer any questions you may have.
If you get any side effects, do not stop taking GAPENTIN without first talking to your doctor or pharmacist.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- dizziness* or light-headedness
- feeling tired or drowsy*
- unfriendliness*
- unusually overactive*
- forgetfulness, loss of concentration or confusion
- difficulty speaking
- changes in your weight*
- constipation, diarrhoea
- nausea and/or vomiting*, indigestion
- dry mouth, red swollen gums
- muscle pain or cramps, back pain
- swelling of the hands or feet
- runny or blocked nose
- fever*
- bronchitis*, lung infection*
- sore throat and discomfort when swallowing, coughing.
These are the more common side effects of GAPENTIN. Mostly these are mild and short-lived.
Tell your doctor as soon as possible if you notice any of the following:
- weakness, unsteadiness when walking including falling, reduced co-ordination or slowed reactions
- unusual changes in mood* or behaviour such as restlessness, nervousness, or excitement
- signs of new onset of, or increased irritability or agitation
- signs of depression
- seeing or hearing things that are not there, irrational thinking
- blurred or double vision, uncontrollable jerky eye movements, difficulty seeing
- signs of frequent infections such as fever, severe chills, sore throat or mouth ulcers
- trouble breathing or shallow breaths (respiratory depression)
- loss of consciousness.
The side effects in the above lists marked * have been specifically reported in children taking GAPENTIN.
Tell your doctor immediately if you experience any of the following:
- suicidal thoughts
- suicide attempts.
Tell your doctor immediately or go to the Accident and Emergency department of your nearest hospital if you have any thoughts of harming yourself or committing suicide.
Tell your doctor immediately or go to Accident and Emergency at your nearest hospital if you notice any of the following:
- more frequent or more severe seizures (fits)
- chest pain, a very fast heart rate
- sudden signs of allergy such as rash (called anaphylactic reactions), itching or hives, fever, swollen lymph gland, swelling of the face, lips, tongue or other parts of the body, shortness of breath, wheezing or difficulty breathing
These are very serious, rare side effects. You may need urgent medical attention or hospitalisation.
Tell your doctor if you notice anything else that is making you feel unwell. Other side effects not listed above may happen in some people. Some of these side effects (for example, changes in thyroid function, structure of bones, high cholesterol or blood pressure) can only be found when your doctor does blood tests from time to time to check your progress.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
After taking it
Storage
Keep your capsule in the pack until it is time to take them. If you take the capsule out of the pack, they may not keep well.
Keep your capsules in a cool dry place where the temperature stays below 25°C.
Do not store GAPENTIN or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car on hot days. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor or pharmacist tells you to stop taking this medicine or the capsules have passed their expiry date, ask your pharmacist what to do with any that are left over.
Product description
What it looks like
GAPENTIN capsules come in 3 strengths:
- GAPENTIN 100mg – white capsule marked with “G 100” in blue ink containing a white crystalline powder.
- GAPENTIN 300mg – yellow capsule marked with “G 300” in blue ink containing a white crystalline powder.
- GAPENTIN 400mg – orange capsule marked with “G 400” in blue ink containing a white crystalline powder.
Each blister pack contains 100 capsules.
Ingredients
Active ingredient:
GAPENTIN capsules come in three strengths and contain either 100 mg, 300 mg or 400 mg of gabapentin.
Inactive ingredients:
The capsules also contain:
- lactose monohydrate
- purified talc
- maize starch
- gelatin
- titanium dioxide
- iron oxide yellow (300 mg & 400 mg capsules only)
- iron oxide red (400 mg capsules only)
- TekPrint SB-6018 blue ink.
Contains sugars as lactose monohydrate.
May contain trace amounts of phenylalanine and sulfites.
Sponsor
Arrow Pharma Pty Ltd
15-17 Chapel Street
Cremorne VIC, 3121
Australian Registration Numbers:
100 mg capsules: AUST R 107472
300 mg capsules: AUST R 107494
400 mg capsules: AUST R 107498
This leaflet was revised in February 2023.
Published by MIMS April 2023



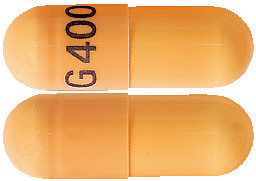
 For patients undergoing haemodialysis who have never received gabapentin, a loading dose of 300 mg to 400 mg is recommended, then 200 mg to 300 mg of gabapentin following each 4 hours of haemodialysis.
For patients undergoing haemodialysis who have never received gabapentin, a loading dose of 300 mg to 400 mg is recommended, then 200 mg to 300 mg of gabapentin following each 4 hours of haemodialysis. The relative risk for suicidal thoughts or behaviour was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
The relative risk for suicidal thoughts or behaviour was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
 Other events in more than 2% of children but equally or more frequent in the placebo group included: pharyngitis, upper respiratory infection, headache, rhinitis, convulsions, diarrhoea, anorexia, coughing, and otitis media.
Other events in more than 2% of children but equally or more frequent in the placebo group included: pharyngitis, upper respiratory infection, headache, rhinitis, convulsions, diarrhoea, anorexia, coughing, and otitis media.
 Chemical name: 1-(aminomethyl) cyclohexaneacetic acid.
Chemical name: 1-(aminomethyl) cyclohexaneacetic acid.