Notes
Distributed by Generic Health Pty Ltd
1 Name of Medicine
Gliclazide.
2 Qualitative and Quantitative Composition
Each Gliclazide Lupin MR tablet contains 60 mg gliclazide.
For the full list of excipients, see Section 6.1 List of Excipients.
3 Pharmaceutical Form
Modified release tablet.
Gliclazide Lupin MR 60 mg tablets.
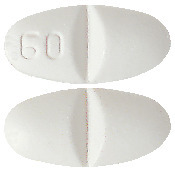
White to off white, oval shaped, biconvex, uncoated tablet with a breakline on both sides and debossed with '60' on one side of the breakline on one surface and plain on the other side.4.1 Therapeutic Indications
Type II diabetes in association with dietary measures when dietary measures alone are inadequate to control blood glucose.
During controlled clinical trials in patients with type II diabetes, a modified release formulation of gliclazide (30 mg-120 mg), taken as a single daily dose, was shown to be effective long term in controlling blood glucose levels, based on monitoring of HbA1c.
4.2 Dose and Method of Administration
For adult use only.
Gliclazide Lupin MR have a break bar and may be administered as whole or as half tablets (see Section 5.2 Pharmacokinetic Properties). So that the modified release properties of the product can be maintained, tablets should not be chewed or crushed.
Whole or half tablets of Gliclazide Lupin MR should be taken with food because there is an increased risk of hypoglycaemia if a meal is taken late, if an inadequate amount of food is consumed or if the food is low in carbohydrate. It is recommended that the medication be taken at breakfast time. If a dose is forgotten, the dose taken on the next day should not be increased.
A single daily dose provides an effective blood glucose control. The daily dose may vary from half a tablet to two tablets per day, i.e. 30 mg to 120 mg taken orally. The initial recommended dose is half a tablet (30 mg), even in elderly patients (≥ 65 years). The daily dose should not exceed two tablets (120 mg).
As with all hypoglycaemic agents, the dose should be titrated according to the individual patient's response. Titration should be carried out in steps of 30 mg, according to the fasting blood glucose response. Each step should last for at least two weeks.
Previously untreated patients should commence with half a tablet of Gliclazide Lupin MR (30 mg) dose and will benefit from dose titration until the appropriate dose is reached.
Gliclazide Lupin MR may be used to replace other anti-diabetic treatments without any transitional period. If a patient is switched from a hypoglycaemic sulphonylurea with a prolonged half-life they should be carefully monitored (for one to two weeks) in order to avoid hypoglycaemia due to possible residual effects of the previous therapy.
Gliclazide Lupin MR may be given in combination with biguanides, alpha glucosidase inhibitors or insulin.
Elderly patients.
The efficacy and tolerance of the modified release formulation of gliclazide (30 mg-120 mg) has been confirmed in clinical trials in patients over 65 years who were given the same dosage regimen as the general population. The dosage is therefore identical to that recommended for adults under the age of 65 years.
Renal impairment.
The efficacy and tolerance of the modified release formulation of gliclazide (30 mg-120 mg) has been confirmed in clinical trials of patients with mild to moderate renal failure (creatinine clearance of between 15 and 80 mL/min) who were given the same dosage regimen as the general population. No dosage adjustment is therefore required in patients with mild to moderate renal impairment. Use of Gliclazide Lupin MR in patients with severe renal impairment is contraindicated (see Section 4.3 Contraindications).4.3 Contraindications
This medication is contra-indicated in the following cases.
Hypersensitivity to gliclazide, other sulphonylureas, sulfonamides, or to any of the excipients.
Type I diabetes, diabetic keto-acidosis, diabetic pre-coma and coma.
Severe renal or hepatic impairment: in these cases the use of insulin is recommended.
Treatment with miconazole (see Section 4.5 Interactions with Other Medicines and Other Forms of Interactions).
Pregnancy and lactation (see Section 4.6 Fertility, Pregnancy and Lactation).
4.4 Special Warnings and Precautions for Use
The risks of hypoglycaemia, together with its symptoms, treatment and conditions that predispose to its development, should be explained to the patient and to family members. The patient should be informed of the importance of following dietary advice, of taking regular exercise, and of regular monitoring of blood glucose levels.
Hypoglycaemia.
Hypoglycaemia may occur following administration of sulphonylureas. Rarely cases may be severe and prolonged. This may involve hospitalisation and glucose infusion may need to be continued for several days.
Careful selection of patients and of the dose used, as well as provision of adequate information to the patient are necessary to avoid hypoglycaemic episodes. The following factors may increase the risk of hypoglycaemia:
patient does not follow the doctor's treatment advice (particularly elderly patients);
malnutrition;
irregular mealtimes, skipping meals, periods of fasting or dietary changes;
imbalance between physical exercise and carbohydrate intake;
renal impairment;
severe hepatic impairment;
overdose of anti-diabetic agents;
certain endocrine disorders: thyroid disorders, hypopituitarism and adrenal impairment, concomitant administration of certain other medicines (see Section 4.5 Interactions with Other Medicines and Other Forms of Interactions).
Gliclazide should only be prescribed if the patient is likely to have a regular food intake (including breakfast). It is important to have a regular carbohydrate intake due to the increased risk of hypoglycaemia if a meal is delayed, an inadequate amount of food is consumed, or the food is low in carbohydrate. Hypoglycaemia is more likely to occur during periods of low-calorie diet, following prolonged or strenuous exercise, following alcohol intake or during treatment with a combination of hypoglycaemic agents.
Poor blood glucose control.
Blood glucose control in treated patients may be affected by St. John's Wort (Hypericum perforatum) preparations (see Section 4.5 Interactions with Other Medicines and Other Forms of Interactions), fever, trauma, infection or surgical intervention. It may be necessary to discontinue treatment and to administer insulin in these cases.
The efficacy of oral anti-diabetic agents often decreases in the long term. This may be due to progression in the severity of the diabetes, or to a reduced response to treatment. This phenomenon is known as secondary failure and should be distinguished from primary failure, when the drug is ineffective as first-line treatment. However, before classifying the patient as a secondary failure, dose adjustment and reinforcement of dietary measures should be considered.
Unstable blood glucose level (dysglycaemia).
Disturbances in blood glucose, including hypoglycaemia and hyperglycaemia have been reported, in diabetic patients receiving concomitant treatment with fluoroquinolones, especially in elderly patients. Indeed, careful monitoring of blood glucose is recommended in all patients receiving gliclazide and a fluoroquinolone at the same time.
Use in renal and hepatic impairment.
Severe renal or hepatic impairment may affect the distribution of gliclazide and hepatic impairment may also reduce the capacity for neoglucogenesis. These two effects increase the risk of severe hypoglycaemic reactions. A hypoglycaemic episode in these patients may be prolonged and appropriate management should be initiated.
Glucose-6-phosphate dehydrogenase deficiency (G6PD).
Treatment of patients with G6PD-deficiency with sulphonylurea agents can lead to haemolytic anaemia. Since gliclazide belongs to the chemical class of sulphonylurea drugs, caution should be used in patients with G6PD-deficiency and a non-sulphonylurea alternative should be considered.
Patients with porphyria.
Cases of acute porphyria have been described with the class of sulfonylurea drugs, in patients who have porphyria.
Use in the elderly.
See Section 4.2 Dose and Method of Administration; Section 5.2 Pharmacokinetic Properties.
Paediatric use.
Not recommended for paediatric use, see Section 4.2 Dose and Method of Administration.
Effects on laboratory tests.
Glycated haemoglobin should be monitored regularly. Blood glucose measurement may also be useful.4.5 Interactions with Other Medicines and Other Forms of Interactions
Blood glucose monitoring during and after treatment is necessary when gliclazide is used with medicines which can interact with gliclazide. It may also be necessary to adjust the dose of gliclazide during and after treatment with such medicines.
1. The following medications are likely to increase the risk of hypoglycaemia.
Concomitant use which is contraindicated. Miconazole (systemic route, oromucosal gel).
Increases the hypoglycaemic effect with possible onset of hypoglycaemia symptoms, or even coma.
Concomitant use which is not recommended. Phenylbutazone (systemic route).
Increases the hypoglycaemic effect of sulphonylureas (displaces their binding to plasma proteins and/or reduces their elimination). It is preferable to use a different anti-inflammatory agent, or else to warn the patient and emphasise the importance of self-monitoring. Where necessary, adjust the dose during and after treatment with the anti-inflammatory agent.
Alcohol.
Acute alcohol intoxication potentiates the hypoglycaemic action of all sulphonylurea agents by inhibiting compensatory reactions. This can lead to the onset of hypoglycaemic coma. Ingestion of alcohol may also cause a disulfiram-like reaction with characteristic flushing of the face, throbbing headache, giddiness, tachypnoea, tachycardia or angina pectoris. Chronic alcohol abuse may, as a result of liver enzyme induction, increase the metabolism of sulphonylurea drugs, shortening the plasma half-life and duration of action.
Avoid alcohol or medicines containing alcohol.
Concomitant use which requires special care. Potentiation of the blood glucose lowering effect and therefore in some instances, hypoglycaemia may occur when one of the following medications is taken.
Other anti-diabetic agents (insulins, acarbose, biguanides, metformin, thiazolidinediones, dipeptidyl peptidase-4 inhibitors, GLP-1 receptor agonists), sulfonamides, clarithromycin, clofibrate, salicylates (high doses), chloramphenicol, MAOIs, β-blockers, H2-receptor antagonists, ACE inhibitors, fluconazole and non-steroidal anti-inflammatory agents.
2. The following medications may cause an increase in blood glucose levels.
Advise the patient and emphasise the importance of glucose monitoring.
Concomitant use which is not recommended. Danazol.
If the use of danazol cannot be avoided, it may be necessary to adjust the dose of gliclazide during and after treatment with danazol.
Concomitant use which requires special care. Chlorpromazine.
High doses (> 100 mg per day of chlorpromazine) can increase blood glucose levels (reduced insulin release). Advise the patient and emphasise the importance of glucose monitoring. It may be necessary to adjust the dose of gliclazide during and after treatment with chlorpromazine.
Glucocorticoids (systemic and local route: intra-articular, cutaneous and rectal preparations) and tetracosactrin.
Concomitant use may increase blood glucose levels with possible ketosis (glucocorticoids cause reduced tolerance to carbohydrates). Emphasise the importance of blood glucose monitoring, particularly at the start of treatment. It may be necessary to adjust the dose of gliclazide during and after treatment with glucocorticoids.
Salbutamol, terbutaline (intravenous).
May cause increased blood glucose levels due to beta-2 agonist effects. If necessary, switch to insulin.
Barbiturates, estrogens and progestogens.
May adversely affect blood sugar control with hypoglycaemic agents in some patients by causing increased blood glucose levels.
St. John's Wort (Hypericum perforatum) preparations.
Gliclazide exposure is decreased by St. John's Wort (Hypericum perforatum).
3. The following products may cause unstable blood glucose.
Concomitant use which requires special care. Fluoroquinolones.
In case of a concomitant use of gliclazide and a fluoroquinolone, the patient should be warned of the risk of unstable blood glucose, and the importance of blood glucose monitoring should be emphasised.
Concomitant use to be taken into consideration. Anticoagulant therapy (warfarin).
Sulphonylureas may lead to potentiation of anticoagulation during concurrent treatment. Adjustment of warfarin may be necessary.4.6 Fertility, Pregnancy and Lactation
Effects on fertility.
No data available.
(Category C)
In animal studies embryo-toxicity and/or birth defects have been demonstrated with some sulfonylureas.
Gliclazide should not be used in pregnant women. From a clinical point of view, there are limited data (less than 300 pregnancies) to allow evaluation of the possible malformative or foetotoxic effects of gliclazide, when administered during pregnancy. Animal studies of gliclazide have not shown any teratogenic effect.
Gliclazide is contraindicated during pregnancy and insulin is the drug of first choice for treatment of diabetes during pregnancy. Treatment should be changed from gliclazide to insulin therapy before pregnancy is attempted, or as soon as pregnancy is discovered. Control of diabetes should be achieved before the time of conception to reduce the risk of congenital abnormalities linked to uncontrolled diabetes.
It is not known whether gliclazide or its metabolites are excreted in breast milk. Given the risk of neonatal hypoglycaemia, gliclazide is contraindicated in women who are breast feeding. A risk to newborns/infants cannot be excluded.4.7 Effects on Ability to Drive and Use Machines
Patients should be made aware of the symptoms of hypoglycaemia and should be careful if driving or operating machinery, especially at the beginning of treatment.
4.8 Adverse Effects (Undesirable Effects)
Good clinical acceptability of gliclazide, has been established in many studies as well as in medical practice.
The safety of a modified release formulation of gliclazide (30 mg-120 mg) has been evaluated in controlled clinical trials in 955 patients, of which 728 patients were treated in long-term comparative trials, against a gliclazide immediate release formulation (80 mg-320 mg), for up to ten months. In these comparative trials, the overall incidence and type of adverse events were similar in both groups. Adverse events were generally mild and transient, not requiring discontinuation of therapy.
However, where patients did discontinue due to adverse events, the percentage was lower in the modified release group (2.9%) than in the immediate release group (4.5%).
Hypoglycaemia (see Section 4.3 Contraindications; Section 4.4 Special Warnings and Precautions for Use).
The most frequent adverse reaction with gliclazide is hypoglycaemia.
As is the case with all sulphonylurea drugs, hypoglycaemic reactions have been reported following gliclazide administration. However, a number of studies have shown that hypoglycaemia is less common with gliclazide than with glibenclamide.
Possible symptoms of hypoglycaemia are: headache, intense hunger, nausea, vomiting, lassitude, sleep disorders, agitation, aggression, poor concentration, reduced awareness and slowed reactions, depression, confusion, visual and speech disorders, aphasia, tremor, paresis, sensory disorders, dizziness, feeling of powerlessness, loss of self-control, delirium, convulsions, shallow respiration, bradycardia, drowsiness and loss of consciousness, possibly resulting in coma and/or death. In addition, signs of adrenergic counter-regulation may be observed: sweating, clammy skin, anxiety, tachycardia, hypertension, palpitations, angina pectoris and cardiac arrhythmia.
Usually, symptoms disappear after intake of carbohydrate such as sugar (artificial sweeteners have no effect). Experience with other sulphonylureas shows that hypoglycaemia can recur even when these measures are initially effective. If a hypoglycaemic episode is severe or prolonged, and even if it is temporarily controlled by intake of sugar, immediate medical treatment or even hospitalisation is required.
In long-term comparative studies, the percentage of patients experiencing hypoglycaemic episodes was similar between patients treated with the modified release formulation of gliclazide (11.6%) and those treated with the immediate release formulation of gliclazide (11.1%). However, the number of hypoglycaemic episodes per 100 patient months was lower in the modified release group (3.5) than in the immediate release group (4.8).
Analysis of elderly patients (over 65 years old) showed less hypoglycaemia than in the general population, with a prevalence of hypoglycaemic episodes lower in the modified release group (2.6 hypoglycaemic episodes for 100 patient months) than in the immediate release group (4.1).
The percentage of patients experiencing hypoglycaemic episodes in the sub-population with renal failure, was similar to that observed in the general population.
Adverse events reported during controlled clinical trials with the modified release formulation of gliclazide were those expected in an ageing population with diabetes.
Adverse events that were reported in at least 2.0% of patients, in long-term controlled clinical studies, are presented in Table 1. The most frequent adverse events were not specifically related to the disease (such as respiratory infections or back pain).
 Analysis of adverse events in sub-populations showed a similar pattern to that seen in the general population. Gender, age and renal impairment had no significant influence on the safety profile of the modified release formulation of gliclazide.
Analysis of adverse events in sub-populations showed a similar pattern to that seen in the general population. Gender, age and renal impairment had no significant influence on the safety profile of the modified release formulation of gliclazide.
Other adverse effects.
Gastrointestinal disturbances (reported with gliclazide), including nausea, dyspepsia, diarrhoea, abdominal pain, vomiting and constipation may be avoided or minimised if gliclazide is taken with breakfast.
The following adverse effects have been rarely reported.
Skin and subcutaneous tissue disorders.
Pruritus, urticaria, maculopapular rashes, rash, angioedema, erythema and bullous reactions (such as Stevens-Johnson Syndrome (SJS) and toxic epidermal necrolysis (TEN) (as with other sulfur-containing medications) and autoimmune bullous disorders and exceptionally, drug rash with eosinophilia and systemic symptoms (DRESS).
Blood and lymphatic system disorders (as with other sulphonylurea medications).
Anaemia, leucopoenia, thrombocytopenia and agranulocytosis. These are in general reversible upon discontinuation of medication.
Hepatobiliary disorders.
Elevations of serum bilirubin and hepatic enzymes (AST, ALT, alkaline phosphatase) levels, and exceptionally, hepatitis (isolated reports). Treatment should be discontinued if cholestatic jaundice appears. These symptoms usually disappear after discontinuation of treatment.
Investigations.
Occasional elevations of serum creatinine, blood urea nitrogen.
Eye disorders.
Transient visual disturbances may occur due to changes in blood glucose levels, particularly on initiation of treatment. As with any glucose-lowering medication, transient visual disturbances may occur on initiation of treatment due to changes in blood glucose levels.
Class effects.
The following adverse events have been observed with sulphonylureas: cases of erythrocytopenia, agranulocytosis, haemolytic anaemia, pancytopenia and allergic vasculitis, hyponatremia, elevated liver enzyme levels and even impairment of liver function (e.g. with cholestasis and jaundice) and hepatitis which regressed after withdrawal of the sulphonylurea or led to life-threatening liver failure in isolated cases.
Reporting suspected adverse effects.
Reporting suspected adverse reactions after registration of the medicinal product is important. It allows continued monitoring of the benefit-risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions at www.tga.gov.au/reporting-problems.4.9 Overdose
Overdose of sulphonylureas may cause hypoglycaemia.
Moderate symptoms of hypoglycaemia (without loss of consciousness or neurological signs), should be corrected by carbohydrate intake, dose adjustment and/or modification of diet. Strict monitoring should be continued until the doctor is sure that the patient is out of danger.
Severe hypoglycaemic reactions are possible (with coma, convulsions or other neurological disorders) and must be treated as a medical emergency, requiring immediate hospitalisation.
If hypoglycaemic coma is diagnosed or suspected, the patient should be given a rapid I.V. injection of 50 mL of concentrated glucose solution (20 to 30%). This should be followed by continuous infusion of a more dilute glucose solution (10%) at a rate necessary to maintain blood glucose levels above 5 mmol/L. It is recommended that patients should be monitored closely for a 48-hour period at least.
Plasma clearance of gliclazide may be prolonged in patients with hepatic disease. However, due to the strong binding of gliclazide to proteins, dialysis is not effective in these patients.
For information on the management of overdose, contact the Poisons Information Centre on 13 11 26 (Australia).
5 Pharmacological Properties
5.1 Pharmacodynamic Properties
Mechanism of action.
Gliclazide reduces blood glucose levels by stimulating insulin secretion from the β-cells of the islets of Langerhans. Gliclazide shows high affinity, strong selectivity and reversible binding to the β-cell KATP channels with a low affinity for cardiac and vascular KATP channels. Increased postprandial insulin and C-peptide secretion persists after two years of treatment.
In type II diabetes, gliclazide restores the first peak of insulin secretion in response to glucose and increases the second phase of insulin secretion. A significant increase in insulin release is seen in response to stimulation induced by a meal or glucose.
Gliclazide also has extra-pancreatic effects and haemovascular properties. It has been shown to increase peripheral insulin sensitivity.
In muscle, euglycaemic hyperinsulinaemic clamp studies with gliclazide have demonstrated significantly increased (35%) insulin mediated glucose uptake which may improve diabetes control. Gliclazide potentiates insulin action on muscle glycogen synthase. These effects are consistent with a post-transcriptional action of gliclazide on GLUT4 glucose transporters.
Studies on glucose turnover have further shown that gliclazide decreases hepatic glucose production, leading to an improvement in fasting blood glucose levels.
Gliclazide has been shown in some studies to have actions independent of that on glucose levels. These haemovascular effects of gliclazide include:
Partial inhibition of platelet aggregation and adhesion with a decrease in markers of platelet activation (beta thromboglobulin, thromboxane B2).
Increased vascular endothelial fibrinolytic activity (increased tPA activity).
Anti-oxidant properties, notably a reduction in plasma lipid peroxides and increased erythrocyte superoxide dismutase activity.
Inhibition of the increased adhesiveness of type II diabetic patient's monocytes to endothelial cells in vitro.
The anti-oxidant, platelet inhibiting and fibrinolytic actions of gliclazide involve processes which have been implicated in the pathogenesis of vascular complications of type II diabetes. There is no clinical evidence that the haemovascular effects of gliclazide are of therapeutic benefit in type II diabetes patients.
Clinical trials.
No data available.
5.2 Pharmacokinetic Properties
Absorption.
Hydration of the tablets induces formation of a gel to activate drug release. Plasma levels increase progressively, resulting in a plateau-shaped curve from the sixth to the twelfth hour after administration. Intra-individual variability is low. Gliclazide is completely absorbed and food intake does not affect the rate or degree of absorption.
Distribution.
Plasma protein binding is approximately 95%. The relationship between the dose administered and the area under the concentration curve as a function of time is linear for doses of gliclazide up to 90 mg/day. At the highest evaluated dose (135 mg/day), the AUC increases slightly more than proportionally to the dose.
Metabolism.
Gliclazide is mainly metabolised in the liver, the products of which are extensively excreted in the urine.
Excretion.
Less than 1% of unchanged drug is recovered in the urine. No active metabolites have been detected in plasma. The clearance of gliclazide has been found to be slightly reduced as a function of age. This reduction, however, is not considered to be clinically significant. The elimination half-life of gliclazide is approximately 16 hours.
Use in elderly.
No clinically significant modifications in the pharmacokinetic parameters have been observed in elderly patients.
5.3 Preclinical Safety Data
Genotoxicity.
No data available.
Carcinogenicity.
No data available.6 Pharmaceutical Particulars
6.1 List of Excipients
Calcium hydrogen phosphate dihydrate, povidone, hypromellose, magnesium stearate.
6.2 Incompatibilities
Incompatibilities were either not assessed or not identified as part of the registration of this medicine.
6.3 Shelf Life
In Australia, information on the shelf life can be found on the public summary of the Australian Register of Therapeutic Goods (ARTG). The expiry date can be found on the packaging.
6.4 Special Precautions for Storage
Store below 25°C.
6.5 Nature and Contents of Container
Gliclazide Lupin MR 60 mg tablets are supplied in PVC/Al or PVC/Aclar/Al blister pack containing either 20 or 60 tablets.*
* Not all pack sizes may be marketed.
Australian registration numbers.
Gliclazide Lupin MR 60 mg tablets: AUST R 370851.
6.6 Special Precautions for Disposal
In Australia, any unused medicine or waste material should be disposed of by taking to your local pharmacy.
6.7 Physicochemical Properties
Gliclazide is an oral hypoglycaemic sulphonylurea which differs from other related compounds by an N-containing heterocyclic ring with an endocyclic bond.
It is a white or almost white powder, practically insoluble in water, freely soluble in dichloromethane, sparingly soluble in acetone and slightly soluble in ethanol 96%. The melting point of gliclazide is approximately 168°C.
Chemical structure.
 Chemical name: 1-(3-azabicyclo[3.3.0]oct-3-yl)-3-p-tolylsulphonylurea.
Chemical name: 1-(3-azabicyclo[3.3.0]oct-3-yl)-3-p-tolylsulphonylurea.
Molecular formula: C15H21N3O3S.
CAS number.
21187-98-4.7 Medicine Schedule (Poisons Standard)
(S4) Prescription Only Medicine.
Summary Table of Changes


 Analysis of adverse events in sub-populations showed a similar pattern to that seen in the general population. Gender, age and renal impairment had no significant influence on the safety profile of the modified release formulation of gliclazide.
Analysis of adverse events in sub-populations showed a similar pattern to that seen in the general population. Gender, age and renal impairment had no significant influence on the safety profile of the modified release formulation of gliclazide. Chemical name: 1-(3-azabicyclo[3.3.0]oct-3-yl)-3-p-tolylsulphonylurea.
Chemical name: 1-(3-azabicyclo[3.3.0]oct-3-yl)-3-p-tolylsulphonylurea.