What is in this leaflet
This leaflet answers some common questions about Glimepiride Sandoz.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking this medicine against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What Glimepiride Sandoz is used for
This medicine is used to control blood glucose (sugar) in patients with type 2 diabetes mellitus.
It contains the active ingredient glimepiride.
Glimepiride belongs to a group of medicines called sulfonylureas.
It works by increasing the amount of insulin produced by your pancreas.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
This medicine is not addictive.
This medicine is available only with a doctor's prescription.
Before you take Glimepiride Sandoz
When you must not take it
Do not take this medicine if you have an allergy to:
- glimepiride, the active ingredient, or to any of the other ingredients listed at the end of this leaflet under Product description
- other sulfonylureas (e.g. Minidiab, Diamicron or Daonil)
- any other similar medicines such as sulfonamides (e.g. sulphur antibiotics or thiazide diuretics).
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin.
Do not take this medicine if you have or have had any of the following medical conditions:
- a previous reaction to glimepiride, other sulfonylureas or sulphonamides
- type 1 diabetes mellitus
- diabetic ketoacidosis
- diabetic coma or pre-coma
- severe kidney disease or if you require dialysis
- severe liver failure
- hereditary problems of galactose intolerance, Lapp lactase deficiency or glucose-galactose malabsorption.
Do not take this medicine if you are pregnant or intend becoming pregnant. It may affect your developing baby if you take it during pregnancy.
Insulin is more suitable for controlling blood glucose during pregnancy. Your doctor will replace Glimepiride with insulin.
Do not take this medicine if you are breastfeeding or plan to breastfeed. The active ingredient in Glimepiride Sandoz passes into breast milk and there is a possibility that your baby may be affected.
Do not give this medicine to children. Safety and effectiveness in children has not been established.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you have or have had any of the following medical conditions:
- kidney problems
- liver problems
- unstable diabetes
- adrenal, pituitary or thyroid problems
- heart failure
- glucose-6-phosphate dehydrogenase (G6PD) deficiency
- diabetic coma.
Tell your doctor if you:
- drink alcohol in any amount
- do not eat regular meals
- do a lot of exercise or heavy exercise or work
- are ill or feeling unwell.
Alcohol, diet, exercise and your general health all strongly affect the control of your diabetes.
If you have not told your doctor about any of the above, tell him/her before you start taking Glimepiride Sandoz.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and Glimepiride Sandoz may interfere with each other. These include:
- alcohol
- other medicines used to treat diabetes such as metformin or insulin
- some medicines used to treat reflux and stomach ulcers
- some anti-inflammatory medicines, such as aspirin, ibuprofen, naproxen or mesalazine
- some medicines used to treat arthritis and gout
- some antibiotics, such as clarithromycin or rifampicin
- anabolic steroids
- oral contraceptives
- corticosteroids, glucagon, thyroid hormones and other hormonal medications
- some cancer and organ transplant treatments
- some cholesterol-lowering and weight reduction medicines
- some antidepressants, psychiatric and sedating medicines
- some medicines used to treat epilepsy
- some medicines used to treat high blood pressure and some heart conditions, such as ACE inhibitors and beta-blockers
- some medicines used to treat blood clots, blood vessel problems and irregular heart rhythms such as coumarin derivatives
- some fluid and glaucoma medications
- large doses of laxatives.
These medicines may be affected by Glimepiride Sandoz or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
Certain medicines such as beta-blockers and clonidine may hide the warning symptoms of hypoglycaemia (low blood glucose).
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
How to take Glimepiride Sandoz
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions, ask your doctor or pharmacist for help.
How much to take
Ask your doctor or pharmacist if you are unsure of the correct dose for you. They will tell you exactly how much to take.
Follow the instructions they give you. If you take the wrong dose, Glimepiride Sandoz may not work as well and your condition may not improve.
Your doctor will tell you how many tablets to take each day. Depending on your blood glucose levels, your doctor may increase or decrease the dose.
How to take it
Swallow the tablets whole with a full glass of water.
When to take Glimepiride Sandoz
Take your medicine at about the same time each day. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
Take your medicine during or immediately after a meal, at about the same time each day.
Glimepiride Sandoz tablets are usually taken once a day, immediately before breakfast. If your breakfast is very light, you may wait until lunch to take the tablet.
Do not skip meals while taking Glimepiride Sandoz.
How long to take Glimepiride Sandoz
Continue taking your medicine for as long as your doctor tells you.
This medicine helps to control your condition, but does not cure it. It is important to keep taking your medicine even if you feel well.
If you forget to take it
Take your dose as soon as you remember, and continue to take it as you would normally.
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Tell your doctor if you have missed several doses.
Missed doses can cause hyperglycaemia (high blood glucose).
Do not take a double dose to make up for the dose that you missed. This may increase the risk of hypoglycaemia (low blood glucose).
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much Glimepiride Sandoz. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
Symptoms of an overdose may include symptoms of hypoglycaemia (low blood glucose), such as:
- weakness, trembling or shaking
- sweating
- lightheadedness, dizziness, headache or lack of concentration
- tearfulness, crying, irritability
- hunger
- numbness around the lips and tongue
- loss of co-ordination or confusion
- slurred speech
- loss of consciousness or seizures.
While you are taking Glimepiride Sandoz
Things you must do
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking Glimepiride Sandoz.
Tell any other doctors, dentists, and pharmacists who treat you that you are taking this medicine.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking this medicine.
If you become pregnant while taking this medicine, tell your doctor immediately.
If you are about to have any blood tests, tell your doctor that you are taking this medicine. It may interfere with the results of some tests.
Keep all of your doctor's appointments so that your progress can be checked.
Your doctor may do some regular tests to make sure the medicine is working and to prevent unwanted side effects. These include:
- blood glucose testing
- checks of your eyes, feet, kidneys, heart, circulation, blood and blood pressure.
Make sure all your friends, relatives, work colleagues or carers know that you have diabetes. Make sure they can recognise the symptoms of hypoglycaemia and know how to treat them. Provide them with the telephone number for your doctor, the Poisons Information Centre (13 11 26) and Emergency Services.
Symptoms of hypoglycaemia can occur suddenly. These may include:
- weakness, trembling or shaking
- sweating
- lightheadedness, dizziness, headache or lack of concentration
- tearfulness, crying, irritability
- hunger
- numbness around the lips and tongue.
Always carry some sugary food or drink with you.
If you experience any of the symptoms of hypoglycaemia, raise your blood glucose quickly by taking one of the following:
- 5-7 jelly beans
- 3 teaspoons of sugar or honey
- 1/2 can of sugar-containing soft drink
- 2-3 concentrated glucose tablets.
Unless you are within 10-15 minutes of your next meal or snack, follow up with extra carbohydrates such as plain biscuits, fruit or milk. Taking this extra carbohydrate will prevent a second drop in your blood glucose level.
If hypoglycaemia is severe, it can result in seizures or a loss of consciousness. In this case, an ambulance should be called immediately.
The risk of hypoglycaemia is increased in the following situations:
- too much Glimepiride Sandoz
- certain other medicines
- too much or unexpected exercise
- delayed meal or snack
- too little food.
If you notice the return of any symptoms you had before starting Glimepiride Sandoz (signs of hyperglycaemia (high blood glucose)), tell your doctor immediately.
Signs of hyperglycaemia may include:
- lethargy or tiredness
- headache
- thirst
- passing large amounts of urine
- blurred vision.
The risk of hyperglycaemia is increased in the following situations:
- uncontrolled diabetes
- illness, infection or stress
- taking less Glimepiride Sandoz than prescribed
- certain other medicines
- too little exercise or sudden immobilisation, e.g. after an accident
- eating more carbohydrates than normal.
If you become ill or experience extra stress, injury, fever, infection or need surgery, tell your doctor.
Things you must not do
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or change the dosage without checking with your doctor.
Do not skip meals while taking Glimepiride Sandoz.
Things to be careful of
Be careful driving or operating machinery until you know how Glimepiride Sandoz affects you. This medicine may cause dizziness, light-headedness and tiredness if your blood glucose levels fall too low. If you have any of these symptoms, do not drive, operate machinery or do anything else that could be dangerous.
Be careful when drinking alcohol while you are taking this medicine. If you drink alcohol, your blood glucose levels may fall and you may experience symptoms of hypoglycaemia.
Hypoglycaemia may slow your reaction time and affect your ability to drive or operate machinery. Drinking alcohol can also make this worse.
Protect your skin when you are in the sun, especially between 10am and 3pm. Glimepiride Sandoz may cause your skin to be more sensitive to sunlight than it is normally. Exposure to sunlight may cause a skin rash, itching, redness or a severe sunburn.
If you become sick with a cold, fever or flu, it is very important to continue taking Glimepiride Sandoz, even if you feel unable to eat your normal meal.
If you have trouble eating solid foods, use sugar-sweetened drinks as a carbohydrate substitute or eat small amounts of bland food. Your doctor, pharmacist or diabetes educator can give you a list of food to eat for sick days.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Glimepiride Sandoz.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
The most common side effect is hypoglycaemia. Please refer to the section "While you are taking Glimepiride Sandoz" for symptoms and treatment.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- stomach upset including nausea (feeling sick) and vomiting
- abdominal pain, discomfort, diarrhoea or feeling of fullness in the stomach
- eye problems, including blurred or double vision.
Mostly, these are mild side effects of the medicine and are short-lived.
Tell your doctor as soon as possible if you notice any of the following:
- yellowing of your skin or eyes, also called jaundice
- signs of anaemia such as tiredness, being short of breath and looking pale
- signs of frequent or worrying infections, such as fever, severe chills, sore throat or mouth ulcers
- bleeding or bruising more easily than normal, or reddish or purplish blotches under the skin
- symptoms of sunburn such as redness, itching, swelling or blistering which may occur more quickly than normal.
The above list includes some serious side effects that may require medical attention. Serious side effects are rare.
If any of the following happen, tell your doctor immediately or go to Accident and Emergency at your nearest hospital:
- skin reactions such as itching, redness or itchy hives-like rash or spots
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body.
The above list includes very serious side effects. You may need urgent medical attention or hospitalisation.
Tell your doctor or pharmacist if you notice anything else that is making you feel unwell. Other side effects not listed above may also occur in some people.
Some side effects (for example changes in liver function or low blood cell counts) can only be found when your doctor does tests from time to time to check your progress.
After taking Glimepiride Sandoz
Storage
Keep your medicine in the original container.
If you take it out of its original container it may not keep well.
Keep your medicine in a cool dry place where the temperature stays below 25°C.
Do not store Glimepiride Sandoz or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car.
Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
Glimepiride Sandoz comes in four types of tablets:
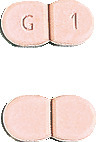
Glimepiride Sandoz 1mg - Light red, flat, modified oblong, scored tablet encoded G|1 on one side.
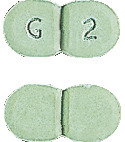
Glimepiride Sandoz 2mg - Light green, flat, modified oblong, scored tablet encoded G|2 on one side.
Glimepiride Sandoz 3mg - Light yellow, flat, modified oblong, scored tablet encoded G|3 on one side.
Glimepiride Sandoz 4mg - Light blue, flat, modified oblong, scored tablet encoded G|4 on one side.
Available in blisters of 30 tablets.
Ingredients
Active ingredients:
- Glimepiride Sandoz 1mg - 1mg glimepiride
- Glimepiride Sandoz 2mg - 2mg glimepiride
- Glimepiride Sandoz 3mg - 3mg glimepiride
- Glimepiride Sandoz 4mg - 4mg glimepiride.
Inactive ingredients:
- lactose
- microcrystalline cellulose
- sodium starch glycollate
- povidone
- magnesium stearate.
Each strength tablet also contains the following colouring agents:
- 1mg - iron oxide red
- 2mg - iron oxide yellow and indigo carmine
- 3mg - iron oxide yellow
- 4mg - indigo carmine.
This medicine does not contain sucrose, gluten, tartrazine or any other azo dyes.
Supplier
Glimepiride Sandoz is supplied in Australia by:
Sandoz Pty Ltd
100 Pacific Highway
North Sydney, NSW 2060
Tel: 1800 726 369
This leaflet was revised in November 2024.
Australian Register Numbers
1mg tablet: AUST R 121781 (blisters)
2mg tablet: AUST R 121786 (blisters)
3mg tablet: AUST R 121788 (blisters)
4mg tablet: AUST R 121790 (blisters)
Published by MIMS February 2025