1 Name of Medicine
The active ingredient is imatinib as the mesilate salt (beta crystals).
2 Qualitative and Quantitative Composition
Each film-coated tablet contains 100 mg or 400 mg of imatinib (equivalent to 119.5 mg or 478 mg imatinib mesilate, respectively).
For the full list of excipients, see Section 6.1 List of Excipients.
3 Pharmaceutical Form
Imatinib Sandoz 100 mg film-coated tablet.
Very dark yellow to brownish orange round tablet with imprint "NVR" on one side and "SA" and score on the other side.
Imatinib Sandoz 400 mg film-coated tablet.
Very dark yellow to brownish orange, ovaloid, biconvex tablet with "400" on one side and score on the other side and "SL" on each side of the score.4.1 Therapeutic Indications
Imatinib Sandoz is indicated for the:
treatment of patients with chronic myeloid leukaemia (CML);
treatment of adult and paediatric patients with newly diagnosed Philadelphia chromosome positive acute lymphoblastic leukaemia (Ph+ ALL) integrated with chemotherapy;
treatment of adult patients with relapsed or refractory Ph+ ALL as monotherapy;
treatment of adult patients with myelodysplastic/myeloproliferative diseases (MDS/MPD) associated with platelet-derived growth factor receptor (PDGFR) gene re-arrangements, where conventional therapies have failed;
treatment of adult patients with aggressive systemic mastocytosis (ASM), where conventional therapies have failed;
treatment of adult patients with hypereosinophilic syndrome (HES) and/or chronic eosinophilic leukaemia (CEL);
treatment of patients with KIT (CD117) positive unresectable and/or metastatic malignant gastrointestinal stromal tumours (GIST);
adjuvant treatment of adult patients at high risk of recurrence following complete gross resection of KIT (CD117)-positive primary GIST (see Section 4.2 Dose and Method of Administration; Section 5.1 Pharmacodynamic Properties, Clinical trials);
treatment of adult patients with unresectable, recurrent and/or metastatic dermatofibrosarcoma protuberans (DFSP).
4.2 Dose and Method of Administration
Therapy should be initiated by a physician experienced in the treatment of patients with haematological malignancies and malignant sarcomas, as appropriate.
The prescribed dose should be administered orally, with a meal and a large glass of water to minimise the risk of gastrointestinal disturbances. Doses of 400 mg or 600 mg should be administered once daily, whereas a daily dose of 800 mg should be administered as 400 mg twice a day, in the morning and in the evening.
For patients unable to swallow the film-coated tablets, the tablets may be dispersed in a glass of water or apple juice. The required number of tablets should be placed in the appropriate volume of beverage (approximately 50 mL for a 100 mg tablet, and 200 mL for a 400 mg tablet) and stirred with a spoon. The suspension should be administered immediately after complete disintegration of the tablet(s).
Treatment should be continued as long as the patient continues to benefit.
Monitoring of response to Imatinib Sandoz therapy in CML patients should be performed routinely and when therapy is modified, to identify suboptimal response, loss of response to therapy, poor patient compliance, or possible drug-drug interaction. Results of monitoring should guide appropriate CML management.
Dosage for CML.
Adults.
The recommended dosage of Imatinib Sandoz is 400 mg/day for patients in chronic phase CML and 600 mg/day for patients in accelerated phase or blast crisis. An increase in dose from 400 mg to 600 mg in patients with chronic phase disease, or from 600 mg to 800 mg (given as 400 mg twice daily) in patients in accelerated phase or blast crisis may be considered in the absence of severe adverse drug reactions and severe non-leukaemia-related neutropenia or thrombocytopenia in the following circumstances: disease progression at any time; failure to achieve a satisfactory haematological response after at least 3 months of treatment; failure to achieve a cytogenetic response after 12 months of treatment; or loss of a previously achieved haematological and/or cytogenetic response.
If, in patients with chronic phase disease escalated to 600 mg per day, the dose was associated with no greater than mild toxicity during the initial 4 weeks, then further dose escalation to 800 mg per day may be considered (see Section 5.1 Pharmacodynamic Properties, Clinical trials).
Dosage for Ph+ ALL.
Adults.
The recommended dose of Imatinib Sandoz is 600 mg/day for adult patients with Ph+ ALL.
Dosage for MDS/MPD.
The recommended initial dose of Imatinib Sandoz is 400 mg/day for adult patients with MDS/MPD. An increase from 400 mg to 600 mg or 800 mg per day may be considered if response is inadequate.
Dosage for ASM.
The recommended initial dose of Imatinib Sandoz is 400 mg/day for adult patients with ASM. An increase from 400 mg to 600 mg or 800 mg per day may be considered if response is inadequate.
For patients with SM associated with eosinophilia, a clonal haematological disease related to the fusion kinase FIP1L1-PDGFRα a starting dose of 100 mg/day is recommended. Dose increase from 100 mg to 400 mg for these patients may be considered in the absence of adverse drug reactions if assessments demonstrate an insufficient response to therapy.
Dosage for HES/CEL.
The recommended initial dose of Imatinib Sandoz is 400 mg/day for adult patients with HES/CEL. An increase from 400 mg to 600 mg or 800 mg per day may be considered if response is inadequate.
For HES/CEL patients with demonstrated FIP1L1-PDGFRα fusion kinase, a starting dose of 100 mg/day is recommended. Dose increase from 100 mg to 400 mg for these patients may be considered in the absence of adverse drug reactions if assessments demonstrate an insufficient response to therapy.
Dosage for GIST.
The recommended dose of Imatinib Sandoz is 400 mg/day or 600 mg/day for adult patients with unresectable and/or metastatic, malignant GIST.
For patients who develop disease progression while receiving 400 mg/day, a dose increase to 800 mg/day may be considered. There are limited data on the efficacy of increasing dose from 600 to 800 mg/day. Treatment with Imatinib Sandoz in GIST patients should be continued until disease progression.
The recommended dose of Imatinib Sandoz is 400 mg/day for the adjuvant treatment of adult patients following resection of GIST. In clinical trials one year of imatinib and three years of imatinib were studied. In the patient population defined in Study SSG XVIII/AIO, three years of imatinib is recommended (see Section 5.1 Pharmacodynamic Properties, Clinical trials). The optimal treatment duration with Imatinib Sandoz is not known.
Dosage for DFSP.
The recommended dose of Imatinib Sandoz is 800 mg/day for adult patients with DFSP.
Dose adjustments for adverse drug reactions.
Non-haematological adverse drug reactions.
If a severe non-haematological adverse drug reaction develops (such as severe hepatotoxicity or severe fluid retention) Imatinib Sandoz should be withheld until the event has resolved. Thereafter, treatment can be resumed as appropriate depending on the initial severity of the event.
If elevations in bilirubin > 3 x institutional upper limit of normal (IULN) or in liver transaminases > 5 x IULN occur, Imatinib Sandoz should be withheld until bilirubin levels have returned to < 1.5 x IULN and transaminase levels to < 2.5 x IULN. Treatment with Imatinib Sandoz may then be continued at a reduced daily dose. In adults the daily dose should be reduced from 400 mg to 300 mg or from 600 mg to 400 mg, or from 800 mg to 600 mg. In children the daily dose should be reduced from 340 mg/m2 to 260 mg/m2.
In adjuvant GIST, if non haematological toxicity of grade 2 is experienced, imatinib should be withheld until the toxicity has resolved to ≤ grade 1. Treatment can be resumed at the previous dose, however, if grade 2 toxicity recurs, treatment should be withheld and then re-introduced at a reduced dose of 300 mg per day. If grade 3/4 toxicity occurs, treatment is withheld until toxicity resolves to ≤ grade 1. The daily dose is resumed at a reduced dose of 300 mg per day.
Haematological adverse drug reactions.
Dose reduction or treatment interruption for severe neutropenia and thrombocytopenia are recommended as indicated in Table 1.

Special populations.
Children.
Dosing for children should be on the basis of body surface area (mg/m2). The dose 340 mg/m2 daily is recommended for children with chronic phase CML and advanced phase CML and Ph+ ALL (not to exceed the total dose of 600 mg daily).
Treatment can be given as a once-daily dose in CML and Ph+ ALL. In CML, alternatively, the daily dose may be split into two administrations - one in the morning and one in the evening.
The dosing recommendation is currently based on a small number of paediatric patients (see Section 5.2 Pharmacokinetic Properties; Section 5.1 Pharmacodynamic Properties, Clinical trials). There is no experience with the use of imatinib in children with CML below 2 years of age and with Ph+ ALL below 1 year of age.
There is very limited to no experience with the use of imatinib in children in other indications.
Recommendations for dose reduction in children are extrapolations based on practice in adults, as data in children are extremely limited (see Table 1).
Hepatic insufficiency.
Imatinib is metabolised by the liver. Patients with mild or moderate liver dysfunction should be given the minimum recommended dose of 400 mg daily, and patients with severe liver dysfunction should start at 300 mg daily (see Section 5.2 Pharmacokinetic Properties). The dose can be reduced if the patient develops unacceptable toxicity (see Section 4.2 Dose and Method of Administration, Dose adjustments for adverse drug reactions).
Renal insufficiency.
Imatinib and its metabolites are not significantly excreted via the kidney. Patients with renal dysfunction could be given the minimum recommended dose of 400 mg daily as starting dose (see Section 5 Pharmacological Properties). However, in these patients caution is recommended. The dose can be reduced if not tolerated. If tolerated, the dose can be increased for lack of efficacy (see Section 4.4 Special Warnings and Precautions for Use).
Elderly patients.
No significant age related pharmacokinetic differences have been observed in adult patients in clinical trials which included over 20% of patients age 65 and older. Decrease in hemoglobin and increased blood creatinine showed a different incidence with age in the adjuvant 36-month trial in GIST. No specific dose recommendation is necessary in the elderly.
Patient monitoring.
Complete blood counts must be performed regularly during therapy with Imatinib Sandoz and liver function (transaminases, bilirubin, alkaline phosphatase) should also be monitored regularly (see Section 4.4 Special Warnings and Precautions for Use, Haematological toxicity, Hepatotoxicity).
Patients should be weighed regularly (see Section 4.4 Special Warnings and Precautions for Use, Fluid retention and oedema). Patients with cardiac disease or risk factors for cardiac failure should be monitored carefully and any patient with signs or symptoms consistent with cardiac failure should be evaluated and treated.4.3 Contraindications
Use in patients with a hypersensitivity to the active substance or to any of the excipients is contraindicated.
4.4 Special Warnings and Precautions for Use
When Imatinib Sandoz is co-administered with other medications, there is a potential for drug interactions. Caution should be used when taking Imatinib Sandoz with rifampicin or other strong CYP3A4 inducers, ketoconazole or other strong CYP3A4 inhibitors, CYP3A4 substrates with a narrow therapeutic window (e.g. ciclosporin or pimozide) or CYP2C9 substrates with a narrow therapeutic window (e.g. warfarin and other coumarin derivatives) (see Section 4.5 Interactions with Other Medicines and Other Forms of Interactions).
Fluid retention and oedema.
Imatinib is often associated with oedema and occasionally serious fluid retention (see Section 4.8 Adverse Effects (Undesirable Effects), Adverse reactions). Patients should be weighed and monitored regularly for signs and symptoms of fluid retention. An unexpected rapid weight gain should be carefully investigated and appropriate treatment provided. The probability of oedema was increased with higher imatinib dose in the CML studies. Severe fluid retention (pleural effusion, pericardial effusion, pulmonary oedema, ascites) was reported in 2 to 8% of patients taking imatinib for CML. In addition, severe superficial oedema was reported in 2-5% of the patients with CML. In clinical trials, there was an increased incidence of these events in elderly patients and those with a prior history of cardiac disease.
Severe superficial oedema and severe fluid retention (pleural effusion, pulmonary oedema and ascites) were reported in 89 patients (11%) taking 400 mg and in 127 (16%) taking 800 mg in the combined Phase III trials of patients taking imatinib for GIST.
Haematological toxicity.
Complete blood counts must be performed regularly during therapy with Imatinib Sandoz. Treatment of CML patients with imatinib has been associated with neutropenia or thrombocytopenia and anaemia. However, the occurrence of these cytopenias is dependent on the stage of the disease being treated and they were more frequent in patients with accelerated phase CML or blast crisis as compared to patients with chronic phase CML. Treatment with Imatinib Sandoz may be interrupted or the dose may be reduced (see Section 4.2 Dose and Method of Administration, Haematological adverse reactions).
Gastrointestinal haemorrhage.
In the Phase III studies in patients with unresectable or metastatic malignant GIST 211 patients (12.9%) reported Grade 3/4 haemorrhage at any site. In the Phase II study in patients with unresectable or metastatic malignant GIST (study B2222), eight patients (5.4%) were reported to have had gastrointestinal (GI) haemorrhage and four patients (2.7%) were reported to have had haemorrhages at the site of tumour deposits. Of these, seven patients (5%) suffered events of grade III/IV severity.
The tumour haemorrhages have been either intra-abdominal or intra-hepatic, depending on the anatomical location of tumour lesions. GI sites of tumour may have contributed to reports of GI bleeding in this patient population. In addition, gastric antral vascular ectasia (GAVE), a rare cause of GI hemorrhage, has been reported in post-marketing experience in patients with CML, ALL and other diseases. Onset of bleeding did not correlate with platelet count, tumour burden or duration of treatment. Fatal haemorrhages have been reported in other studies of imatinib in GIST.
Patients should therefore be monitored for gastrointestinal symptoms at the start of and during therapy with Imatinib Sandoz. When needed, Imatinib Sandoz discontinuation may be considered.
Tumour lysis syndrome.
Cases of tumour lysis syndrome (TLS) have been reported in patients treated with imatinib. Due to possible occurrence of TLS, correction of clinically significant dehydration and treatment of high uric acid levels are recommended prior to initiation of Imatinib Sandoz (see Section 4.8 Adverse Effects (Undesirable Effects)).
Thrombotic microangiopathy.
BCR-ABL tyrosine kinase inhibitors (TKIs) have been associated with thrombotic microangiopathy (TMA), including individual case reports for imatinib (see Section 4.8 Adverse Effects (Undesirable Effects)). If laboratory or clinical findings associated with TMA occur in a patient receiving Imatinib Sandoz, treatment should be discontinued and thorough evaluation for TMA, including ADAMTS13 activity and anti-ADAMTS13-antibody determination, should be completed. If anti-ADAMTS13-antibody is elevated in conjunction with low ADAMTS13 activity, treatment with Imatinib Sandoz should not be resumed.
Hepatitis B reactivation.
Reactivation of hepatitis B can occur in patients who are chronic carriers of this virus after receiving a BCR-ABL tyrosine kinase inhibitor (TKI), such as imatinib. Some cases of hepatitis B reactivation involving drugs of the BCR-ABL TKI class resulted in acute hepatic failure or fulminant hepatitis leading to liver transplantation or a fatal outcome (see Section 4.8 Adverse Effects (Undesirable Effects)).
Patients should be tested for hepatitis B infection before initiating treatment with imatinib. Patients currently on imatinib should have baseline testing for hepatitis B infection in order to identify chronic carriers of the virus. Experts in liver disease and in the treatment of hepatitis B should be consulted before treatment is initiated in patients with positive hepatitis B serology (including those with active disease) and for patients who test positive for hepatitis B infection during treatment. Carriers of hepatitis B virus who require treatment with imatinib should be closely monitored for signs and symptoms of active hepatitis B infection throughout therapy and for several months following termination of therapy.
Use in hepatic impairment.
Cases of liver injury, including hepatic failure and hepatic necrosis, have been observed with imatinib (see Section 4.8 Adverse Effects (Undesirable Effects)). Liver function (transaminases, bilirubin, alkaline phosphatase) should be monitored regularly in patients receiving Imatinib Sandoz. Laboratory abnormalities should be managed with interruption of treatment and/or dose reduction (see Section 4.2 Dose and Method of Administration, Non-haematological adverse reactions).
In patients with hepatic dysfunction (mild, moderate or severe), peripheral blood counts and liver enzymes should be carefully monitored (see Section 4.8 Adverse Effects (Undesirable Effects)). It is not known whether Imatinib Sandoz can exacerbate existing liver dysfunction.
When imatinib is combined with high dose chemotherapy regimens, an increase in serious hepatic reactions has been detected. Hepatic function should be carefully monitored in circumstances where Imatinib Sandoz is combined with chemotherapy regimens also known to be associated with hepatic dysfunction (see Section 4.8 Adverse Effects (Undesirable Effects)).
One patient, who was taking paracetamol regularly for fever, died of acute liver failure. Although the aetiology is currently unknown, special caution should be exercised when using paracetamol. Patients should be warned to avoid or restrict the use of over-the-counter and prescription medicines containing paracetamol.
Severe congestive heart failure and left ventricular dysfunction.
Severe congestive heart failure and left ventricular dysfunction have occasionally been reported in patients taking imatinib. Most of the patients with reported cardiac events have had other co-morbidities and risk factors, including advanced age and previous medical history of cardiac disease. In an international randomised phase III study in 1106 patients with newly diagnosed Ph+ CML in chronic phase, severe cardiac failure and left ventricular dysfunction were observed in 0.7% of patients taking imatinib compared to 0.9% of patients taking IFN + Ara-C.
Patients with cardiac disease or renal failure.
Patients with cardiac disease, risk factors for cardiac failure or history of renal failure should be monitored carefully and any patient with signs or symptoms consistent with cardiac failure or renal failure should be evaluated and treated.
Hypereosinophilic cardiac toxicity.
In patients with hypereosinophilic syndrome (HES) with occult infiltration of HES cells within the myocardium, isolated cases of cardiogenic shock/left ventricular dysfunction have been associated with HES cell degranulation upon the initiation of imatinib therapy. The condition was reported to be reversible with the administration of systemic steroids, circulatory support measures and temporarily withholding imatinib. Myelodysplastic (MDS)/myeloproliferative (MPD) diseases and systemic mastocytosis may be associated with high eosinophil levels. Performance of an echocardiogram and determination of serum troponin should therefore be considered in patients with HES/CEL, and in patients with MDS/MPD or SM associated with high eosinophil levels. If either is abnormal, the prophylactic use of systemic steroids (1-2 mg/kg) for one to two weeks concomitantly with Imatinib Sandoz should be considered at the initiation of therapy.
Hypothyroidism.
Clinical cases of hypothyroidism have been reported in thyroidectomy patients undergoing levothyroxine replacement during treatment with Imatinib Sandoz. Thyroid stimulating hormone (TSH) levels should be closely monitored in such patients.
Use in renal impairment.
Long-term treatment with imatinib may be associated with a clinically significant decline in renal function. Renal function should, therefore, be evaluated prior to the start of imatinib therapy and closely monitored during therapy, with particular attention to those patients exhibiting risk factors for renal dysfunction. If renal dysfunction is observed, appropriate management and treatment should be initiated in accordance with standard treatment guidelines.
Imatinib Sandoz and its metabolites are not excreted via the kidney to a significant extent. Patients with mild and moderate impairment of renal function appear to have a higher plasma exposure than patients with normal renal function. The increase is approximately 1.5- to 2-fold, corresponding to a 1.5-fold elevation of plasma AGP, to which imatinib binds strongly. The free drug clearance of imatinib is probably similar between patients with renal impairment and those with normal renal function, since renal excretion represents only a minor elimination pathway for imatinib (see Section 4.2 Dose and Method of Administration; Section 5 Pharmacological Properties).
Use in the elderly.
See Section 4.2 Dose and Method of Administration, Special populations, Elderly patients.
Paediatric use.
Imatinib efficacy and safety have been demonstrated in children with Ph+ chronic phase CML and in Ph+ALL. There is no experience with imatinib in children with accelerated phase CML below 2 years of age and with Ph+ALL below 1 year of age, and very limited experience in children with blast crisis. There is very limited experience with imatinib in children under 3 years of age (see Section 5.1 Pharmacodynamic Properties, Clinical trials, Paediatric patients).
There have been case reports of growth retardation occurring in children and pre-adolescents receiving Imatinib Sandoz. The long term effects of prolonged treatment with imatinib on growth in children are unknown. Therefore, close monitoring of growth in children under imatinib treatment is recommended.
Effects on laboratory tests.
Interference of imatinib with laboratory and/or diagnostic tests has not been studied.
Toxicities from long-term use.
Because follow-up of most patients treated with imatinib is relatively short (< 6 months), it is important to consider potential toxicities suggested by animal studies, specifically, liver and kidney toxicity and immunosuppression (see Section 5.3 Preclinical Safety Data).
Phototoxicity.
Exposure to direct sunlight should be avoided or minimised due to the risk of phototoxicity associated with imatinib treatment. Patients should be advised to protect skin from sunlight with protective clothing and high sun protection factor (SPF) sunscreen; and avoid sunlamps and tanning beds.4.5 Interactions with Other Medicines and Other Forms of Interactions
Drugs that may alter imatinib plasma concentrations.
Drugs that may increase imatinib plasma concentrations.
Substances that inhibit the cytochrome P450 isoenzyme CYP3A4 activity could decrease metabolism and increase imatinib concentrations. There was a significant increase in exposure to imatinib (the mean Cmax and AUC of imatinib rose by 26% and 40%, respectively) in healthy subjects when it was co-administered with a single dose of ketoconazole (a CYP3A4 inhibitor). Caution should be taken when administering Imatinib Sandoz with inhibitors of the CYP3A4 family (e.g. ketoconazole, erythromycin, clarithromycin, itraconazole, HIV antivirals, grapefruit juice).
Drugs that may decrease imatinib plasma concentrations.
Substances that are inducers of CYP3A4 activity (e.g. dexamethasone, phenytoin, carbamazepine, rifampicin, phenobarbital (phenobarbitone), or St. John's wort [Hypericum perforatum] may significantly reduce exposure to Imatinib Sandoz. Pretreatment of 14 healthy volunteers with multiple doses of rifampicin, 600 mg daily for 8 days, followed by a single 400 mg dose of imatinib, increased the oral-dose clearance of imatinib by 3.8-fold (90% confidence interval = 3.5- to 4.3-fold). This represents mean decreases in Cmax, AUC(0-24) and AUC(0-∞) by 54%, 68% and 74% of the respective values without rifampicin treatment. Similar results were observed in patients with malignant gliomas treated with imatinib while taking enzyme-inducing anti-epileptic drugs (EIADEDs) such as carbamazepine, oxcarbamazepine, phenytoin, fosphenytoin, phenobarbital (phenobarbitone) and primidone. The plasma AUC for imatinib decreased by 73% compared to patients not on EIAEDs. In two published studies, concomitant administration of imatinib and a product containing St. John's wort led to a 30-32% reduction in the AUC of imatinib.
Co-administration of rifampicin or other CYP3A4 inducers should be avoided wherever possible. In patients where rifampicin or other CYP3A4 inducers are indicated, alternative therapeutic agents with less enzyme induction potential should be considered.
Drugs that may have their plasma concentration altered by Imatinib Sandoz.
Imatinib increases the mean Cmax and AUC of simvastatin (CYP3A4 substrate) 2- and 3.5-fold, respectively, indicating an inhibition of the CYP3A4 by imatinib. Therefore, caution is recommended when administering Imatinib Sandoz with CYP3A4 substrates with a narrow therapeutic window (e.g. ciclosporin, pimozide). Imatinib Sandoz will increase plasma concentrations of other CYP3A4 metabolised drugs (e.g. triazole-benzodiazepines, dihydropyridine calcium channel blockers, certain HMG-CoA reductase inhibitors, i.e. statins, etc).
In vitro, imatinib inhibits the cytochrome P450 isoenzyme CYP2D6 activity at concentrations similar to those that affect CYP3A4 activity. Imatinib at 400 mg twice daily had a weak inhibitory effect on CYP2D6-mediated metoprolol metabolism, with metoprolol Cmax and AUC being increased by approximately 23%. Co-administration of imatinib with CYP2D6 substrates, such as metoprolol, does not seem to be a risk factor for drug-drug interactions and dose adjustment may not be necessary. Commonly used drugs metabolised by CYP2D6 include some antidepressants, neuroleptics and anti-arrhythmic agents.
In vitro data suggest that imatinib has some capacity to act as an inhibitor of CYP2C9 and CYP2C19, although at concentrations higher than would be expected in plasma with recommended doses. However, caution should be exercised with the concomitant use of drugs metabolised by CYP2C9, especially those with a narrow therapeutic window (e.g. warfarin and other coumarin derivatives). PT prolongation was observed following co-administration with warfarin. In view of the potential interaction between imatinib and warfarin, the international normalised ratio (INR) of patients who require anticoagulation with warfarin should be monitored closely, especially when Imatinib Sandoz dose adjustments are necessary. Consideration should be given to anticoagulation with low-molecular weight heparin or unfractionated heparin.
Imatinib inhibits paracetamol O-glucuronidation in vitro with Ki value of 58.5 micromol/L. The inhibition was not observed in vivo in Korean CML patients after co-administration of imatinib (400 mg/day between days two and eight) and paracetamol (1000 mg/day on day eight). Imatinib pharmacokinetics were not altered by paracetamol. Exercise caution in non-Korean patients when co-administering Imatinib Sandoz and paracetamol. Also exercise caution with concomitant use of Imatinib Sandoz at doses > 400 mg/day or the chronic use of concomitant paracetamol and Imatinib Sandoz (see Section 4.4 Special Warnings and Precautions for Use).4.6 Fertility, Pregnancy and Lactation
Effects on fertility.
In a study of fertility, in which male rats were dosed orally for 70 days prior to mating and female rats were dosed for 14 days prior to mating and through to gestational day 6, testicular and epididymal weights and percent motile sperm were decreased at 60 mg/kg (approximately equal to the maximum clinical dose of 800 mg/day, based on body surface area). This was not seen at doses ≤ 20 mg/kg (one-fourth the maximum human dose of 800 mg). There was no effect on mating or on number of pregnant females. At 60 mg/kg, but not at doses ≤ 20 mg/kg, female rats had significant post-implantation fetal loss and a reduced number of live fetuses.
Human studies on male patients receiving imatinib and its effect on male fertility and spermatogenesis have not been performed. Male patients concerned about their fertility on Imatinib Sandoz treatment should consult with their physician.
(Category D)
Women of childbearing potential must be advised to use highly effective contraception during treatment (methods that result in less than 1% pregnancy rate) and for at least 15 days after stopping treatment with Imatinib Sandoz.
There are no clinical trials on the use of imatinib in pregnant women. There have been postmarketing reports of spontaneous abortions and infant congenital anomalies from women who have taken imatinib.
Imatinib Sandoz should be used during pregnancy only if the expected benefit outweighs the potential risk to the foetus. If Imatinib Sandoz is used during pregnancy, or if the patient becomes pregnant while taking Imatinib Sandoz, the patient should be apprised of the potential risk to the foetus. Imatinib mesilate was teratogenic in rats when administered during organogenesis at oral doses ≥ 30 mg/kg, approximately equal to one third the maximum clinical dose of 800 mg/day, based on body surface area. Teratogenic effects included exencephaly or encephalocele, protruded tongue, absent/reduced frontal and absent parietal bones. Female rats also experienced significant post-implantation loss in the form of early fetal resorptions at doses > 30 mg/kg, with total fetal loss in all animals at doses > 100 mg/kg.
Administration of imatinib to rats from early gestation through weaning at an oral dose of 45 mg/kg/day (approximately half the maximal clinical dose of 800 mg/day based on body surface area) resulted in an increase in post-implantation loss. Additionally, F1 offspring showed reproductive effects (including decreased numbers of implantation sites, increased numbers of resorptions and decreased numbers of viable foetuses). Mild maternotoxicity was also observed at this dose.
Both imatinib and its active metabolite can be distributed into human milk. The milk plasma ratio was determined to be 0.5 for imatinib and 0.9 for the metabolite, suggesting greater distribution of the metabolite into the milk. Considering the combined concentration of imatinib and of the metabolite and the maximum daily milk intake by infants the total exposure would be expected to be low (~10% of a therapeutic dose). However, since the effects of low-dose exposure of the infant to imatinib are unknown, women taking Imatinib Sandoz should not breastfeed. Administration of imatinib to rats from early gestation through weaning at an oral dose of 45 mg/kg/day (approximately half the maximal clinical dose of 800 mg/day based on body surface area) resulted in delayed preputial separation and a decrease in pup viability (day 0-4 postpartum).4.7 Effects on Ability to Drive and Use Machines
Reports of motor vehicle accidents have been reported in patients receiving imatinib. While most of these reports are not suspected to be caused by imatinib, patients should be advised that they may experience undesirable effects such as dizziness, blurred vision or somnolence during treatment with Imatinib Sandoz. Therefore, caution should be recommended when driving a car or operating machinery.
4.8 Adverse Effects (Undesirable Effects)
Summary of the safety profile.
The overall safety profile of imatinib in human clinical use has been well-characterized through more than 12 years of imatinib experience. During clinical development, the majority of patients experienced adverse events at some point in time. The most frequently reported ADRs (> 10%) were neutropenia, thrombocytopenia, anaemia, headache, dyspepsia, oedema, weight increased, nausea, vomiting, muscle cramps, musculoskeletal pain, diarrhea, rash, fatigue, and abdominal pain. Events were of mild to moderate grade, and only 2 to 5% of patients permanently discontinued therapy due to drug-related event.
The safety profile of imatinib in adult and paediatric patients with Ph+ Leukaemias is similar in regards to the types of events experienced.
In the GIST study B2222, the percentage of some adverse reactions was higher in the patients with newly-diagnosed CML in whom the dose was increased to 800 mg daily compared to the population of patients before dose increase. These more frequent adverse reactions included gastrointestinal haemorrhages, conjunctivitis and elevation of transaminases or bilirubin.
The differences in the safety profile between Ph+ leukaemias and solid tumours are a higher incidence and severity of myelosuppression in Ph+ leukaemias, and GI and intratumoral haemorrhages in GIST patients and are probably due to disease related factors. The most frequent event by system organ class in CML and GIST treatment were GI disorders, skin and subcutaneous tissue disorders, general disorders and administration site conditions, and musculoskeletal and connective tissue disorders.
Other GI conditions, such as gastrointestinal obstruction, perforation and ulceration, appear to be more indication-specific. Other prominent adverse events that have been observed after exposure to imatinib, and which may be causally related, include hepatotoxicity, acute renal failure, hypophosphataemia, severe respiratory adverse reactions, and tumour lysis syndrome and growth retardation in children.
Depending on severity of events, dose adjustment may be required. In very few cases will the medication have to be discontinued based on ADRs.
Adverse reactions.
Adverse reactions with suspected relationship, reported as more than an isolated case, are listed below by system organ class and by frequency. Frequencies are defined as: very common (≥ 1/10), common (≥ 1/100 to < 1/10), uncommon (≥ 1/1000 to < 1/100), rare (≤ 1/10,000 to < 1/1000), very rare (< 1/10,000) including isolated reports. Adverse reactions and their frequencies reported below are based on the registration studies for CML and GIST.
Adverse reactions in clinical studies for CML and GIST. Blood and lymphatic system disorders.
Very common: neutropenia, thrombocytopenia, anaemia.
Common: pancytopenia, febrile neutropenia.
Uncommon: thrombocythaemia, lymphopenia, bone marrow depression, eosinophilia, lymphadenopathy.
Rare: haemolytic anaemia.
Body as a whole.
Very common: fluid retention and oedema, fatigue, weight increased.
Common: pyrexia, weakness, rigors, anasarca, chills, weight decreased.
Uncommon: chest pain, malaise, blood creatine phosphokinase increased, blood lactate dehydrogenase increased.
Rare: blood amylase increased.
Cardiac disorders.
Uncommon: cardiac failure congestive1, pulmonary oedema, palpitations, tachycardia.
Rare: pericardial effusion, arrhythmia, atrial fibrillation, cardiac arrest, myocardial infarction, angina pectoris.
Ear and labyrinth disorders.
Uncommon: vertigo, tinnitus, hearing loss.
Eye disorders.
Common: eyelid oedema, conjunctivitis, lacrimation increased, conjunctival haemorrhage, dry eye, vision blurred.
Uncommon: eye irritation, eye pain, orbital oedema, scleral haemorrhage, retinal haemorrhage, blepharitis, macular oedema.
Rare: cataract, papilloedema, glaucoma.
Gastrointestinal disorders.
Very common: nausea, vomiting, diarrhoea, dyspepsia, abdominal pain2.
Common: abdominal distension, flatulence, constipation, gastro-oesophageal reflux, dry mouth, gastritis.
Uncommon: stomatitis, mouth ulceration, gastrointestinal haemorrhage3, melaena, ascites, gastric ulcer, oesophagitis, haematemesis, cheilitis, dysphagia, pancreatitis.
Rare: colitis, ileus, inflammatory bowel disease.
Hepato-biliary disorders.
Very common: increased hepatic enzymes.
Common: hyperbilirubinaemia.
Uncommon: jaundice, hepatitis, blood alkaline phosphatase increased.
Rare: hepatic failure4, hepatic necrosis4.
Infections and infestations.
Very common: upper respiratory tract infection.
Uncommon: sepsis, nasopharyngitis, pneumonia5, sinusitis, cellulitis, herpes simplex, herpes zoster, influenza, urinary tract infection, gastroenteritis.
Rare: fungal infection.
Metabolism and nutrition disorders.
Common: anorexia.
Uncommon: dehydration, hyperuricaemia, hypokalaemia, appetite increased, appetite decreased, gout, hypophosphataemia, hypercalcaemia, hyperglycaemia, hyponatraemia.
Rare: hyperkalaemia, hypomagnesaemia.
Musculoskeletal, connective tissue and bone disorders.
Very common: muscle spasm and cramps, musculoskeletal pain including myalgia, arthralgia, bone pain6.
Common: joint swelling.
Uncommon: joint and muscle stiffness.
Rare: muscular weakness, arthritis.
Nervous system disorders.
Very common: headache7.
Common: dizziness, taste disturbance, paraesthesia, hypoaesthesia.
Uncommon: cerebral haemorrhage, syncope, peripheral neuropathy, somnolence, migraine, memory impairment, sciatica, restless leg syndrome, tremor.
Rare: optic neuritis, increased intracranial pressure, convulsions.
Psychiatric disorders.
Common: insomnia.
Uncommon: depression, anxiety, libido decreased.
Rare: confusion.
Renal and urinary disorders.
Very common: blood creatinine increased8.
Uncommon: renal failure, renal pain, urinary frequency increased, haematuria.
Reproductive system and breast disorders.
Uncommon: gynaecomastia, erectile dysfunction, breast enlargement, scrotal oedema, menorrhagia, nipple pain, menstruation irregular, sexual dysfunction.
Respiratory, thoracic and mediastinal disorders.
Common: epistaxis, dyspnoea, cough.
Uncommon: pleural effusion9, pharyngolaryngeal pain, pharyngitis.
Rare: pulmonary fibrosis, pleuritic pain, pulmonary hypertension, pulmonary haemorrhage.
Skin and subcutaneous tissue disorders.
Very common: periorbital oedema, dermatitis/eczema/rash.
Common: face oedema, pruritus, erythema, dry skin, alopecia, night sweats, photosensitivity reaction.
Uncommon: petechiae, sweating increased, urticaria, ecchymosis, increased tendency to bruise, onycholysis, folliculitis, purpura, contusion, hypotrichosis, rash pustular, skin hypopigmentation, skin hyperpigmentation, psoriasis, exfoliative dermatitis, bullous eruptions.
Rare: erythema multiforme, leucocytoclastic vasculitis, vesicular rash, Stevens-Johnson syndrome, acute febrile neutrophilic dermatosis (Sweet's syndrome), nail discolouration, angioneurotic oedema, acute generalised exanthematous pustulosis (AGEP).
Vascular disorders.
Common: flushing10, haemorrhage.
Uncommon: haematoma, subdural haematoma, hypertension, hypotension, peripheral coldness, Raynaud's phenomenon.
Rare: thrombosis/embolism.
1 On a patient-year basis, cardiac events including congestive heart failure were more commonly observed in patients with transformed CML than in patients with chronic CML.
2, 3 Abdominal pain and gastrointestinal haemorrhage were most commonly observed in patients with CML than in GIST patients.
4 Some fatal cases of hepatic failure and hepatic necrosis have been reported.
5 Pneumonia was reported most commonly in patients with transformed CML and in patients with GIST.
6 Musculoskeletal pain and related events were more commonly observed in patients with CML than in GIST patients.
7 Headache was most common in GIST patients.
8 Most of the blood creatinine increases were of mild severity (grade 1).
9 Pleural effusion was reported more commonly in patients with GIST and in patients with transformed CML (CML-AP and CML-BC) than in patients with chronic CML.
10 Flushing was most common in GIST patients and bleeding (haematoma, haemorrhage) was most common in patients with GIST and with transformed CML (CML-AP and CML-BC).
Adverse events in CML clinical trials in adults. Adverse events, regardless of relationship to study drug, that were reported in at least 10% of the patients treated in the Phase III study of imatinib versus IFN+Ara-C (Study 0106) and in the single-arm CML studies 0102, 0109 and 0110 are shown in Tables 2 and 3, respectively.

 Adverse events in paediatric CML clinical trials. Experience is limited in patients aged ≤ 18 years treated with imatinib in clinical trials. In these trials, the toxicity of imatinib appeared comparable to that seen in adults. Toxicities included grade 3 or 4 cytopenias involving neutropenia and thrombocytopenia and anaemia. These generally occur within the first several months of therapy.
Adverse events in paediatric CML clinical trials. Experience is limited in patients aged ≤ 18 years treated with imatinib in clinical trials. In these trials, the toxicity of imatinib appeared comparable to that seen in adults. Toxicities included grade 3 or 4 cytopenias involving neutropenia and thrombocytopenia and anaemia. These generally occur within the first several months of therapy.
Adverse events in the unresectable and/or malignant metastatic GIST clinical trial. Adverse events, regardless of relationship to study drug, that were reported in at least 10% of patients treated in the GIST study are shown in Table 4.
 Adverse events in the adjuvant treatment of GIST clinical trials. In study Z9001, the majority of both imatinib and placebo treated patients experienced at least one adverse reaction at some time. The most frequently reported adverse reactions were similar to those reported in other clinical studies in other patient populations and include diarrhoea, fatigue, nausea, oedema, decreased haemoglobin, rash, vomiting and abdominal pain. No new adverse reactions were reported in the adjuvant GIST treatment setting that had not been previously reported in other patient populations including patients with unresectable and/or malignant metastatic GIST. Drug was discontinued for adverse reactions in 57 patients (17%) and 11 patients (3%) of the imatinib and placebo treated patients respectively. Oedema, gastrointestinal disturbances (nausea, vomiting, abdominal distension and diarrhoea), fatigue, low haemoglobin and rash were the most frequently reported adverse reactions at the time of discontinuation.
Adverse events in the adjuvant treatment of GIST clinical trials. In study Z9001, the majority of both imatinib and placebo treated patients experienced at least one adverse reaction at some time. The most frequently reported adverse reactions were similar to those reported in other clinical studies in other patient populations and include diarrhoea, fatigue, nausea, oedema, decreased haemoglobin, rash, vomiting and abdominal pain. No new adverse reactions were reported in the adjuvant GIST treatment setting that had not been previously reported in other patient populations including patients with unresectable and/or malignant metastatic GIST. Drug was discontinued for adverse reactions in 57 patients (17%) and 11 patients (3%) of the imatinib and placebo treated patients respectively. Oedema, gastrointestinal disturbances (nausea, vomiting, abdominal distension and diarrhoea), fatigue, low haemoglobin and rash were the most frequently reported adverse reactions at the time of discontinuation.
Adverse events, regardless of relationship to study drug, that were reported in at least 10% of the patients treated with imatinib are shown in Table 5.
 The adjuvant trial SSG XVIII/AIO compared 1 year and 3 years imatinib treatment. Table 6 shows adverse events, regardless of relationship to study drug, that were reported in at least 5% of patients treated with imatinib. There was a higher incidence of severe (Grade 3-4) and serious adverse events in the 3-year group than the 1-year group, but this was expected because of the longer duration of treatment. Discontinuations due to adverse events were also greater in the 3-year group (13.6%) than the 1-year group (7.7%). Adverse events were generally consistent with the known safety profile of imatinib. There was insufficient data to assess long-term safety.
The adjuvant trial SSG XVIII/AIO compared 1 year and 3 years imatinib treatment. Table 6 shows adverse events, regardless of relationship to study drug, that were reported in at least 5% of patients treated with imatinib. There was a higher incidence of severe (Grade 3-4) and serious adverse events in the 3-year group than the 1-year group, but this was expected because of the longer duration of treatment. Discontinuations due to adverse events were also greater in the 3-year group (13.6%) than the 1-year group (7.7%). Adverse events were generally consistent with the known safety profile of imatinib. There was insufficient data to assess long-term safety.
 Adverse events with imatinib observed in trials in Ph+ ALL, MDS/MPD, SM, HES/CEL and DFSP were generally consistent with those seen in CML and GIST studies.
Adverse events with imatinib observed in trials in Ph+ ALL, MDS/MPD, SM, HES/CEL and DFSP were generally consistent with those seen in CML and GIST studies.
Description of selected adverse drug reactions.
Myelosuppression.
Myelosuppression is very common in cancer patients treated with imatinib.
Myelosuppression, thrombocytopenia, neutropenia and anaemia were the most frequently reported Grade 3 and 4 laboratory abnormalities. Overall, the myelosuppression experienced with imatinib in CML patients was generally reversible and in most patients did not result in dose interruption or dose reduction. Few patients required drug discontinuation. Other events of pancytopenia, lymphopenia and bone marrow depression have also been reported.
Haematologic depression appeared greatest at the highest doses and also appeared to be dependent on stage of CML disease, with Grade 3 or 4 neutropenia and thrombocytopenia between 4 and 6 times higher in blast and accelerated phase (44% and 63%, respectively) as compared to newly diagnosed patients in CP CML (16.7% and 8.9%, respectively). These events can usually be managed with either a reduction of the dose or an interruption of treatment with Imatinib Sandoz, but rarely require discontinuation. The incidence of hematologic toxicities is less in patients with solid tumours (i.e. GIST) than in patients with Ph+ leukaemias, with Grade 3/4 neutropenia and thrombocytopenia occurring approximately 5% and 0%, respectively of GIST patients.
Haemorrhage.
CNS and GI haemorrhages are not uncommon in CML patients with compromised marrow function at baseline. Haemorrhages are well-recognized part of the disease complications in an acutely ill population of leukemic patients, and may result from thrombocytopenia, or less commonly, platelet dysfunction. However, not all patients experiencing CNS and GI haemorrhages during therapy with imatinib are thrombocytopenic.
The most common manifestation of clinically significant bleeding was GI haemorrhage, which occurred most commonly in advanced CML patients and in metastatic GIST patients, where bleeding might occur as part of the underlying disease due to tumour bleeding from tumour haemorrhage/tumour necrosis. In first line CML and in adjuvant GIST setting, the observed frequencies of GI haemorrhage were generally the lowest. Gastric antral vascular ectasia (GAVE) is also rarely reported with imatinib use in the post-marketing setting.
Oedema and fluid retention.
Oedema is a common toxicity of imatinib appearing in greater than 50% of all patients across all indications. Oedema is dose-related and there appears to be a correlation with its occurrence and plasma levels. The most common manifestation is periorbital oedema and somewhat less common is lower extremity oedema. Specific therapy is not usually required. Other fluid retention events occur much less commonly, but due to the location of the anatomic site may be potentially serious. The most frequent fluid retention event was pleural effusion, most commonly observed in advanced CML and metastatic GIST patients. The frequency of cardiac failure was generally low in patients with oedema and fluid retention. It was higher in advanced CML than in other groups. This could be explained by the worse medical condition of advanced CML patients. The same trend was observed for renal failure in patients with oedema and fluid retention. Most patients with oedema and fluid retention were elderly (> 65 years old).
In a clinical study, the frequency of events suggesting congestive heart failure was 1.5% on imatinib vs. 1.1% on IFN-alpha in patients with newly-diagnosed CML. The frequency was appreciably higher in patients with transformed CML (accelerated phase or blast crisis), higher age, or with a baseline haemoglobin of less than 8 g/dL. Across all indications a higher frequency of CHF events observed in patients with CML than in patients with GIST might indicate differences of some of these disease related risk factors. In addition, a recently published special safety analysis of cardiac events within the EORTC study of 942 patients with unresectable or metastatic GIST concluded that imatinib does not induce left ventricular failure in GIST patients where the observed rate was approximately 0.2% while it can be up to 2% in a population with pre-existing cardiac disease.
Skin rashes and severe cutaneous adverse reactions.
A generalized erythematous, maculopapular, pruritic skin rash that can fade despite continued therapy, has been reported. Some patients may have pruritus without accompanying rash, and sometimes there is an exfoliative component. Re-exposure in some patients has resulted in reappearance of rash, but not in all patients. These eruptions generally respond to antihistamines and topical steroids. Occasionally, systemic steroids are required.
Skin rashes have been observed in up to one third of patients treated with imatinib across all indications. These are frequently pruritic and most commonly appear as erythematous, maculopapular or exfoliative lesions on the forearm, the trunk or the face or generalized with systemic expression. Skin biopsies have revealed a toxic drug reaction with a mixed cellular infiltrate. Although most rashes are mild and self-limiting more severe rare cases such as Stevens-Johnson toxic epidermal necrolysis, erythema multiforme or drug rash with eosinophilia and systemic symptoms (DRESS) may require interruption or discontinuation of treatment. Not surprisingly skin reactions were seen at a higher rate than placebo in the adjuvant GIST trial.
Hepatotoxicity.
Hepatotoxicity, occasionally severe, may occur, and has been observed preclinically and clinically. LFT abnormalities usually consisted of mild elevations in transaminases, although a minority of patients had elevated levels of bilirubin. Onset is generally within the first two months of therapy, but has occurred as late as 6 to 12 months after commencing therapy. The levels generally normalize after withholding therapy for 1 to 4 weeks.
Hypophosphatemia.
Low serum phosphate and hypophosphatemia (up to Grade 3/4) has been observed relatively commonly across all indications, however the origin and the clinical significance of this finding have not been established. Imatinib has been shown to inhibit the differentiation of human monocytes into osteoclasts. The decrease was accompanied by a decrease in the resorptive capacity of these cells. A dose-dependent decrease of RANK-L was observed in osteoclasts in the presence of imatinib. Sustained inhibition of osteoclastic activity may lead to counter regulatory response resulting in increased levels of PTH. The clinical relevance of the preclinical findings is yet unclear and an association with skeletal AEs such as bone fractures has not been demonstrated.
In the clinical development program serum phosphate was not routinely measured in all studies. Although it was initially hypothesized that hypophosphatemia might be dose-dependent, 24 month interpretable results from the Phase III TOPS study designed to investigate dose dependency of safety endpoints in patients with newly diagnosed CML, have shown that Grade 3/4 decreased serum phosphate or serum calcium has been experienced by 19.1% vs.15.5% and 5.1% vs. 0.9% of patients receiving 400 mg and 800 mg, respectively.
Gastrointestinal obstruction, perforation or ulceration.
GI ulceration, which may represent in extreme cases local irritation by imatinib, has been observed in a small proportion of patients across all indications. Tumour haemorrhage/tumour necrosis, obstruction and GI perforation seem to be disease-related and have occurred exclusively or more frequently amongst GIST patients. In the case of metastatic GIST, tumour necrosis may occur in the context of tumour response, rarely leading to perforation. GI obstruction/ileus occurred most commonly in the GIST population where it may be caused by tumour obstruction from metastatic GIST and in the adjuvant setting by adhesions from previous GI surgery.
Tumour lysis syndrome.
A causal relationship between tumour lysis syndrome and imatinib treatment is deemed possible, although some cases were confounded by concomitant medications and other independent risks (see Section 4.4 Special Warnings and Precautions for Use).
Growth retardation in children.
Imatinib appears to affect the stature of children, especially children who are pre-pubertal. A causal relationship between growth retardation in children and imatinib treatment could not be ruled out although for some cases of growth retardation there was limited information (see Section 4.4 Special Warnings and Precautions for Use).
Severe respiratory adverse drug reaction.
Severe respiratory events, sometimes fatal, have been observed with imatinib treatment, including acute respiratory failure, pulmonary hypertension, interstitial lung disease and pulmonary fibrosis. Pre-existing cardiac or pulmonary conditions that may be associated with severe respiratory events have been reported in many of these cases.
Laboratory test abnormalities.
In adult patients with CML, cytopenias, particularly neutropenia and thrombocytopenia, have been a consistent finding in all studies, with the suggestion of a higher frequency at high doses ≥ 750 mg (Phase I study). However, the occurrence of cytopenias was also clearly dependent on the stage of the disease (see Table 3 and 4). In patients with newly diagnosed CML, cytopenias were less frequent than in the other CML patients, the frequency of Grade 3 or 4 neutropenias (ANC < 1.0 x 109/L) and thrombocytopenias (platelet count < 50 x 109/L) being between 4 and 6 times higher in blast crisis and accelerated phase as compared to newly diagnosed patients in chronic phase CML (see Table 7 and 8). In the randomised Phase III study in newly diagnosed CML patients, the median time to onset of neutropenia and thrombocytopenia was 44 days (range 7-463) and 43 days (range 8-421), respectively. The median duration of the neutropenic and thrombocytopenic episodes usually ranged from 2 to 3 weeks, and from 3 to 4 weeks, respectively. These events can usually be managed with either a reduction of the dose or an interruption of treatment with Imatinib Sandoz, but can in rare cases lead to permanent discontinuation of treatment.
In paediatric CML patients the most frequent toxicities observed were Grade 3 or 4 cytopenias involving neutropenia, thrombocytopenia and anaemia. These generally occur within the first several months of therapy.
In patients with unresectable or metastatic malignant GIST (study B2222), Grade 3 and 4 anaemias were reported in 5.4% and 0.7% of patients, respectively, and may have been related to gastrointestinal or intra-tumoural bleeding in at least some of these patients. Grade 3 and 4 neutropenias were seen in 7.5% and 2.7% of patients, respectively, and Grade 3 thrombocytopenia in 0.7% of patients. No patient developed Grade 4 thrombocytopenia (see Table 9). The decreases in WBC and neutrophil counts occurred mainly during the first six weeks of therapy, with values remaining relatively stable thereafter.
In patients with CML, grade 3 or 4 elevations of serum transaminases was common and grade 3 or 4 elevation of serum bilirubin was uncommon (see Tables 7-8), except for patients with CML in accelerated phase or blast crisis, where grade 3 elevations were common. The median duration of these episodes was approximately one week. Treatment was discontinued permanently because of liver laboratory abnormalities in less than 0.5% of CML patients.
In patients with advanced or metastatic GIST, grade 3 or 4 elevations of serum transaminases or bilirubin were common (Table 9). In patients with GIST treated in the adjuvant setting, Grade 3 or 4 elevations of serum transaminases was common and grade 3 or 4 elevations of serum bilirubin was absent (Table 10).
Grade 3 or 4 elevations of serum transaminases or bilirubin are usually managed with dose reduction or interruption. There have been cases of cytolytic and cholestatic hepatitis and hepatic failure, some of which have been fatal (see Section 4.4 Special Warnings and Precautions for Use, Use in hepatic impairment).



 Laboratory abnormalities with imatinib observed in trials in Ph+ ALL, MDS/MPD, SM, HES/CEL and DFSP were generally consistent with those seen in CML and GIST studies.
Laboratory abnormalities with imatinib observed in trials in Ph+ ALL, MDS/MPD, SM, HES/CEL and DFSP were generally consistent with those seen in CML and GIST studies.
The following lists the types of ADRs that have been reported from post-marketing experience and from additional clinical studies with imatinib. They include spontaneous case reports as well as serious ADRs from smaller or ongoing clinical studies and the expanded access programmes. Because these ADRs are reported from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to imatinib exposure.
Adverse reactions from post-marketing reports.
Infections and infestations.
Not known: hepatitis B reactivation.
Nervous system disorders.
Uncommon: cerebral oedema.
Eye disorders.
Rare: vitreous haemorrhage.
Cardiac disorders.
Rare: pericarditis, cardiac tamponade.
Vascular disorders.
Uncommon: thrombosis/embolism.
Very rare: anaphylactic shock.
Respiratory, thoracic and mediastinal disorders.
Uncommon: acute respiratory failure1, interstitial lung disease.
Gastrointestinal disorders.
Uncommon: ileus/intestinal obstruction, tumour haemorrhage/tumour necrosis, gastrointestinal perforation2.
Rare: diverticulitis, gastric antral vascular ectasia (GAVE).
Skin and subcutaneous tissue disorders.
Uncommon: palmar-plantar erythrodysaesthesia syndrome, panniculitis (including erythema nodosum).
Rare: lichenoid keratosis, lichen planus, pemphigus.
Very rare: toxic epidermal necrolysis.
Not known: Drug rash with eosinophilia and systemic symptoms (DRESS), pseudoporphyria.
Musculoskeletal and connective tissue disorders.
Very common: musculoskeletal pain upon treatment discontinuation (including myalgia, pain in extremity, arthralgia, bone pain, spinal pain).
Uncommon: osteonecrosis.
Rare: rhabdomyolysis/myopathy.
Unknown: growth retardation in children.
Reproductive disorders.
Very rare: haemorrhagic corpus luteum/haemorrhagic ovarian cyst.
Neoplasm benign, malignant and unspecified (including cysts and polyps).
Rare: tumour lysis syndrome.
Renal and urinary disorders.
Unknown: chronic renal failure.
Blood and lymphatic disorders.
Rare: thrombotic microangiopathy.
1 Fatal cases have been reported in patients with advanced disease, severe infections, severe neutropenia and other serious concomitant conditions.
2 Some fatal cases of gastrointestinal perforation have been reported.
Reporting suspected adverse effects.
Reporting suspected adverse reactions after registration of the medicinal product is important. It allows continued monitoring of the benefit-risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions at www.tga.gov.au/reporting-problems.4.9 Overdose
Experience with higher than therapeutic doses is limited. Isolated cases of imatinib overdosage have been reported spontaneously and in the literature. Generally the reported outcome in these cases was improvement or recovery. In the event of overdosage the patient should be observed and appropriate symptomatic treatment should be given.
Events that have been reported at different dose ranges are as follows:
Adult overdose.
1,200 to 1,600 mg (duration varying between 1 to 10 days): nausea, vomiting, diarrhoea, rash, erythema, oedema, swelling, fatigue, muscle spasms, thrombocytopenia, pancytopenia, abdominal pain, headache, decreased appetite.
1,800 to 3,200 mg (as high as 3,200 mg daily for 6 days): weakness, myalgia, increased CPK, increased bilirubin, gastrointestinal pain.
6,400 mg (single dose): one case in the literature reported one patient who experienced nausea, vomiting, abdominal pain, pyrexia, facial swelling, neutrophil count decreased, increased transaminases.
8 to 10 g (single dose): vomiting and gastrointestinal pain have been reported.
Paediatric overdose.
One 3 year-old male exposed to a single dose of 400 mg experienced vomiting, diarrhoea and anorexia and another 3 year old male exposed to a single dose of 980 mg dose experienced decreased white blood cell count and diarrhoea.
For information on the management of overdose, contact the Poisons Information Centre on 13 11 26 (Australia).5 Pharmacological Properties
ATC code.
Pharmacotherapeutic group: BRC-ABL-tyrosine kinase inhibitor, ATC code: L01EA01.
5.1 Pharmacodynamic Properties
Imatinib mesilate is a protein-tyrosine kinase inhibitor that inhibits the BCR-ABL tyrosine kinase, the constitutive abnormal tyrosine kinase created by the Philadelphia chromosome abnormality in chronic myeloid leukaemia (CML). It inhibits proliferation and induces apoptosis in BCR-ABL positive cell lines, as well as fresh leukaemic cells from CML patients. In colony formation assays using peripheral blood and bone marrow samples from CML patients, imatinib shows inhibition of formation of BCR-ABL positive colonies.
In vitro studies demonstrate imatinib is not entirely selective; it also inhibits the receptor tyrosine kinases for platelet-derived growth factor (PDGF) and stem cell factor (SCF), KIT, and inhibits PDGF- and SCF-mediated cellular events. In vitro, imatinib inhibits proliferation and induces apoptosis in gastrointestinal stromal tumour (GIST) cells, which express an activating KIT mutation. Constitutive activation of the PDGFR or the Abl protein tyrosine kinases as a consequence of fusion to diverse partner proteins or constitutive production of PDGF have been implicated in the pathogenesis of myelodysplastic/myeloproliferative diseases (MDS/MPD), hypereosinophilic syndrome and/or chronic eosinophilic leukaemia (HES/CEL) and dermatofibrosarcoma protuberans (DFSP). In addition constitutive activation of KIT or PDGFR has been implicated in the pathogenesis of systemic mastocytosis (SM). Imatinib inhibits signalling and proliferation of cells driven by dysregulated PDGFR, KIT and ABL kinase activity.
Mechanism of action.
Imatinib is a small molecule protein-tyrosine kinase inhibitor that potently inhibits the activity of the BCR-ABL tyrosine kinase (TK), as well as several receptor TKs: KIT, the receptor for stem cell factor (SCF) coded for by the KIT proto-oncogene, the discoidin domain receptors (DDR1) and DDR2), the colony stimulating factor receptor (CSF-1R) and the platelet-derived growth factor receptors alpha and beta (PDGFR-alpha and PDGFR-beta). Imatinib can also inhibit cellular events mediated by activation of these receptor kinases.
Clinical trials.
Clinical studies in chronic myeloid leukaemia (CML). One large, open-label, multicentre, international randomised Phase III study has been conducted in patients with newly diagnosed Philadelphia chromosome positive (Ph+) chronic myeloid leukaemia (CML). Three international, open-label, single-arm studies have also been conducted in patients with Ph+ CML: 1) in the chronic phase after failure of interferon-alfa (IFN) therapy, 2) in accelerated phase disease, or 3) in myeloid blast crisis. In the open-label studies, about 45% of patients were women and 6% were black, 38-40% of patients were ≥ 60 years of age and 10-12% of patients were ≥ 70 years of age. In addition, children have been treated in two phase I studies.
Chronic phase, newly diagnosed (study 0106).
This phase III study compared treatment with either single-agent imatinib or a combination of interferon-alfa (IFN) plus cytarabine (Ara-C). The main inclusion criteria were: patients aged between 18 and 70 years, diagnosis of chronic phase CML within the previous 6 months, presence of Philadelphia chromosome or variants on cytogenetic analysis, no previous treatment except hydroxyurea or anagrelide. Patients who had failed bone marrow transplantation or who had residual leukaemia after transplantation were excluded from the study. Patients showing lack of response [lack of complete haematological response (CHR) at 6 months, increasing white blood cell count (WBC), no major cytogenetic response (MCyR) at 24 months, loss of response (loss of CHR or MCyR) or severe intolerance to treatment] were allowed to crossover to the alternative treatment arm. In the imatinib arm, patients were treated with 400 mg daily. In the IFN+Ara-C arm, patients were treated with a target dose of IFN of 5 MIU/m2/day subcutaneously in combination with subcutaneous Ara-C 20 mg/m2/day for 10 days/month.
A total of 1106 patients were randomised from 177 centres in 16 countries, 553 to each arm. Baseline characteristics were well balanced between the two arms. Median age was 51 years (range 18-70 years), with 21.9% of patients ≥ 60 years of age. There were 59% males and 41% females; 89.9% Caucasian and 4.7% black patients. At the cut-off for this analysis (7 years after the last patient had been recruited), the median follow-up for all patients was 82 and 80 months in the imatinib and IFN+Ara-C arms, respectively. 60% of patients randomised to imatinib are still receiving first-line treatment. In these patients, the average dose of imatinib was 403 ± 57 mg. The median duration of second-line treatment with imatinib was 64 months. As a consequence of a higher rate of both discontinuations and crossovers, only 2% of patients randomised to IFN+Ara-C are still on first-line treatment. In the IFN+Ara-C arm, withdrawal of consent (14%) was the most frequent reason for discontinuation of first-line therapy, and the most frequent reason for crossover to the imatinib arm was severe intolerance to treatment (26%) and progression (14%).
The primary efficacy endpoint was progression-free survival. Progression was defined as any of the following event: progression to accelerated phase or blast crisis (AP/BC), death, loss of CHR or MCyR or, in patients not achieving a CHR, an increasing WBC despite appropriate therapeutic management. Major cytogenetic response, haematological response, molecular response (evaluation of minimal residual disease), time to accelerated phase or blast crisis and survival are main secondary endpoints. Response data are shown in Table 11.
 Rates of complete haematological response, major cytogenetic response and complete cytogenetic
response on first-line treatment were estimated using the Kaplan-Meier approach, for which non-responses were censored at the date of last examination. Using this approach the estimated cumulative response rates for first-line treatment with imatinib are shown in Table 12.
Rates of complete haematological response, major cytogenetic response and complete cytogenetic
response on first-line treatment were estimated using the Kaplan-Meier approach, for which non-responses were censored at the date of last examination. Using this approach the estimated cumulative response rates for first-line treatment with imatinib are shown in Table 12.
 For analysis of long-term outcomes patients randomised to receive imatinib were compared with patients randomised to receive IFN. Patients who crossed over prior to progression were not censored at the time of crossover, and events that occurred in these patients following crossover were attributed to the original randomised treatment.
For analysis of long-term outcomes patients randomised to receive imatinib were compared with patients randomised to receive IFN. Patients who crossed over prior to progression were not censored at the time of crossover, and events that occurred in these patients following crossover were attributed to the original randomised treatment.
With 7 years follow-up, there were 93 (16.8%) progression events in the imatinib arm: 37 (6.7%) involving progression to AP/BC, 31 (5.6%) loss of MCyR, 15 (2.7%) loss of CHR or increase in WBC and 10 (1.8%) CML unrelated deaths. In contrast, there were 165 (29.8%) events in the IFN+Ara-C arm of which 130 occurred during first-line treatment with IFN+Ara-C.
The estimated rate of progression-free survival at 84 months is 81.2% with 95% CI (78, 85) in the imatinib arm and 60.6% (56,5) in the control arm (p < 0.001) (Figure 1). The yearly rates of progression for imatinib were 3.3% in the 1st year after start of study, 7.5% in the 2nd year and 4.8%, 1.7%, 0.8%, 0.3% and 2.0% in the 3rd, 4th, 5th, 6th and 7th year of study respectively.
The estimated rate of patients free of progression to accelerated phase or blast crisis at 84 months was significantly higher in the imatinib arm compared to the IFN arm (92.5% [90,95] versus 85.1% [82,89], p < 0.001) (Figure 2). The annual rate of progression decreased with time on therapy: yearly rates of disease progression to accelerated phase or blast crisis were 1.5%, 2.8%, 1.6%, 0.9%, 0.5%, 0% and 0.4% in the first to seventh year, respectively.

 A total of 71 (12.8%) and 85 (15.4%) patients died in the imatinib and IFN+Ara-C groups, respectively. At 84 months the estimated overall survival is 86.4% (83, 90) vs. 83.3% (80, 87) in the randomised imatinib and the IFN+Ara-C groups, respectively (p=0.073, log-rank test). This time-to-event endpoint is strongly affected by the high crossover rate from IFN+Ara-C to imatinib. Additionally, a greater number of patients received bone marrow transplant (BMT) after discontinuation of study treatment in the IFN+Ara-C group (n=66, 38 after crossover to imatinib) compared with the imatinib group (n=50, 8 after crossover to IFN) at the 84 month update. When censoring the 48 deaths that occurred after BMT, the 84-months survival rates were 89.6 vs 88.1 (p=0.200, log-rank test). Only 31 deaths (before BMT) of the imatinib patients (5.6%) were attributed to CML, compared to 40 of the IFN+Ara-C patients (7.2%). When only considering these CML-related deaths and censoring any deaths after BMT or due to other reasons, the estimated 84-months survival rates were 93.6% vs. 91.1% (p=0.1, log rank test). The effect of imatinib treatment on survival in chronic phase, newly diagnosed CML has been further examined in a retrospective analysis of the above reported imatinib data with the primary data from another Phase III study using IFN+Ara-C (n=325) in an identical regimen. In this publication, the superiority of imatinib over IFN+Ara-C in overall survival was demonstrated (p < 0.001); within 42 months, 47 (8.5%) imatinib patients and 63 (19.4%) IFN+Ara-C patients had died.
A total of 71 (12.8%) and 85 (15.4%) patients died in the imatinib and IFN+Ara-C groups, respectively. At 84 months the estimated overall survival is 86.4% (83, 90) vs. 83.3% (80, 87) in the randomised imatinib and the IFN+Ara-C groups, respectively (p=0.073, log-rank test). This time-to-event endpoint is strongly affected by the high crossover rate from IFN+Ara-C to imatinib. Additionally, a greater number of patients received bone marrow transplant (BMT) after discontinuation of study treatment in the IFN+Ara-C group (n=66, 38 after crossover to imatinib) compared with the imatinib group (n=50, 8 after crossover to IFN) at the 84 month update. When censoring the 48 deaths that occurred after BMT, the 84-months survival rates were 89.6 vs 88.1 (p=0.200, log-rank test). Only 31 deaths (before BMT) of the imatinib patients (5.6%) were attributed to CML, compared to 40 of the IFN+Ara-C patients (7.2%). When only considering these CML-related deaths and censoring any deaths after BMT or due to other reasons, the estimated 84-months survival rates were 93.6% vs. 91.1% (p=0.1, log rank test). The effect of imatinib treatment on survival in chronic phase, newly diagnosed CML has been further examined in a retrospective analysis of the above reported imatinib data with the primary data from another Phase III study using IFN+Ara-C (n=325) in an identical regimen. In this publication, the superiority of imatinib over IFN+Ara-C in overall survival was demonstrated (p < 0.001); within 42 months, 47 (8.5%) imatinib patients and 63 (19.4%) IFN+Ara-C patients had died.
The degree of cytogenetic response had a clear effect on long-term outcomes in patients on imatinib. Whereas an estimated 96% (93%) of patients with CCyR (PCyR) at 12 months were free of progression to AP/BC at 84 months, only 81% of patients without MCyR at 12 months were free of progression to advanced CML at 84 months (p < 0.001 overall, p = 0.25 between CCyR and PCyR). Based on the 18-months landmark, the estimates were 99%, 90% and 83% respectively, now also including a statistically significant difference between CCyR and PCyR (p < 0.001).
Molecular monitoring represented important additional prognostic information. For patients with CCyR and reduction in BCR-ABL transcripts of at least 3 logarithms at 12 months, the probability of remaining progression free at 60 months was numerically greater when compared to patients who had CCyR but less than 3 log reduction (95% vs. 89%, p=0.068), and significantly greater than that observed for patients who were not in CCyR at 12 months (70%, p < 0.001). Considering only progression to AP/BC, the estimated rates without event were 100%, 95% and 88% respectively (p < 0.001 overall, p=0.007 between CCyR with and without MMR). Using the 18-months landmark, the estimated rates without AP/BC at 60 months were 100% for patients with CCyR and MMR, 98% for patients with CCyR but without MMR and only 87% for patients without CCyR (p < 0.001 overall, p=0.105 between CCyR with and without MMR).
Quality of life was measured using the validated FACT-BRM instrument. All domains were assessed and reported significantly higher scores in the imatinib arm compared to the IFN arm. QoL data showed that patients maintain their well being while on treatment with imatinib.
Escalation of the imatinib dose to 600 mg per day was allowed in patients satisfying one of the following criteria:
No CHR after 3 months;
No minor cytogenetic response (> 65% Ph+) after 12 months; or
Confirmed loss of MCyR (increase in Ph+ bone marrow cells by at least 30 percentage points e.g. from 20% to 50% or from 30% to 60%) confirmed by a second cytogenetic analysis ≥ 1 month later.
If toxicity was no greater than mild during the initial 4 weeks on 600 mg per day, then further dose escalation to 800 mg per day was allowed.
It should be noted, however, that some patients in the above categories eventually responded to imatinib without need for dose escalation. The small number of patients who received a higher dose achieved proportionately more cytogenetic responses (Table 13). Dose escalation was associated with an increased incidence of adverse reactions including gastrointestinal haemorrhage, elevation of serum bilirubin and transaminases and conjunctivitis.

Chronic phase, prior interferon-alfa treatment (study 0110).
532 patients were treated at a starting dose of 400 mg; dose escalation to 600 mg was allowed. The patients were distributed in three main categories according to their response to prior interferon-alfa: failure to achieve (within 6 months) or loss of a complete haematological response (29%), failure to achieve (within 1 year) or loss of a major cytogenetic response (35%), or intolerance to interferon-alfa (36%). Patients had received a median of 14 months of prior IFN therapy at doses ≥ 25 x 106 IU/week and were all in late chronic phase, with a median time from diagnosis of 32 months. Effectiveness was evaluated on the basis of the rate of haematological response and by bone marrow exams to assess the rate of major cytogenetic response (up to 35% Ph+ metaphases) or complete cytogenetic response (0% Ph+ metaphases). Efficacy results are reported in Table 14.
Results were similar in the three subgroups described above.
Accelerated phase (study 0109).
235 patients with accelerated phase disease were enrolled. These patients met one or more of the following criteria: ≥ 15% - < 30% blasts in PB or BM; ≥ 30% blasts + promyelocytes in PB or BM; ≥ 20% basophils in PB; < 100 x 109/L platelets. The first 77 patients were started at 400 mg, with the remaining 158 patients starting at 600 mg.
Effectiveness was evaluated primarily on the basis of the rate of haematological response, reported as either complete haematological response, no evidence of leukaemia (i.e. clearance of blasts from the marrow and the blood, but without a full peripheral blood recovery as for complete responses) or return to chronic phase CML. Cytogenetic responses were also evaluated. Efficacy results are reported in Table 14. Haematological and major cytogenetic responses were more frequent for patients receiving 600 mg imatinib daily than for those receiving 400 mg daily (75% vs 65% and 32% vs 19%, respectively). In accelerated phase, median duration of haematological response for the 400 mg group is 17 months, whereas it is 30.7 months for the 600 mg group (p=0.0027). For the patients treated at 600 mg, the current estimates for median progression-free survival and overall survival are 22.9 and 42.5 months, respectively.
Myeloid blast crisis (study 0102).
260 patients with myeloid blast crisis were enrolled. These patients had ≥ 30% blasts in PB or BM and/or extramedullary involvement other than spleen or liver; 95 (37%) had received prior chemotherapy for treatment of either accelerated phase or blast crisis ("pre-treated patients") whereas 165 (63%) had not ("untreated patients"). The first 37 patients were started at 400 mg; the remaining 223 patients were started at 600 mg.
Effectiveness was evaluated primarily on the basis of rate of haematological response, reported as either complete haematological response, no evidence of leukaemia, or return to chronic phase CML using the same criteria as for the study in accelerated phase. Cytogenetic responses were also assessed. Efficacy results are reported in Table 14. The haematological response rate was higher in untreated patients than in treated patients (36% and 22%, respectively; p=0.0255) and in the group receiving an initial dose of 600 mg than 400 mg (33% and 16%, respectively; p=0.053).
 Efficacy results were similar in men and women and in patients younger and older than age 65. Responses were seen in black patients, but there were too few black patients to allow a quantitative comparison.
Efficacy results were similar in men and women and in patients younger and older than age 65. Responses were seen in black patients, but there were too few black patients to allow a quantitative comparison.
Paediatric patients.
A total of 51 paediatric patients with newly diagnosed and untreated CML in chronic phase were enrolled in an open-label, multicentre, single arm phase II trial and were treated with imatinib 340 mg/m2/day. Imatinib treatment induced a rapid response in newly diagnosed paediatric CML patients with a CHR of 78% after 8 weeks of therapy and a complete cytogenetic response (CCyR) of 65% (comparable to results in adults) after 3 to 10 months of treatment.
In an uncontrolled trial, 14 children with chronic phase CML were treated with imatinib following relapse after stem cell transplantation or resistance to interferon. After excluding 2 children with minimal disease at study entry and another with no cytogenetic data, 6 children (55%) had complete cytogenetic responses and 3 (27%) had major responses. The children were aged 8-20 years with 5 males and 6 females. By dose, the complete response rates were 67% (2 out of 3), 67% (2 out of 3), 33% (1 out of 3) and 50% (1 out of 2) for the 260, 340, 440 and 570 mg/m2 doses respectively. Median follow-up time was 17 months. There are insufficient data to suggest that the lowest dose (260 mg/m2) differs in efficacy compared with the higher doses.
The trial also enrolled 4 children with blast crisis (aged 3-18 years, 3 male), but none with accelerated phase CML. Two children were dosed at 260 mg/m2, one at 340 mg/m2 and one at 570 mg/m2. Based on the criterion of < 5% blasts in bone marrow, neither of the two children treated at 260 mg/m2 responded, but the children treated at the higher doses did. The child treated at 340 mg/m2 achieved a major cytogenetic response. There were no complete responses. The data supports the dose recommendation of 340 mg/m2 for children with blast crisis.
In another uncontrolled trial, there were 3 children with chronic phase CML and one with CML in blast crisis. The 3 chronic phase children (aged 5-13 years, 2 male) received imatinib doses of 200, 242 and 258 mg/m2. Complete cytogenetic responses were achieved in 2 of the 3 children (doses 242, 258 mg/m2). The child with blast crisis, a boy aged 7 years, was treated at 362 mg/m2 and achieved a complete cytogenetic response.
Clinical studies in acute lymphoblastic leukaemia (Ph+ALL). A total of 851 Ph+ ALL patients with either newly diagnosed or relapsed/refractory disease were enrolled in eleven clinical studies, ten were uncontrolled and one was randomized. Of the 851 patients, 93 were paediatric patients (including 4 patients older than 18 and younger than 22 years) treated in one open-label, multicenter, non-randomized phase III study.
Newly diagnosed Ph+ ALL.
Imatinib monotherapy (600 mg per day) as induction was tested in a randomised, controlled study versus chemotherapy (GMALL protocol for elderly patients) in 55 newly diagnosed patients aged 55 years and over. Used as a single agent, imatinib (N= 28) resulted in a significantly higher CHR than chemotherapy (N=27) (96.3% vs. 50%; p=0.0001) in the induction phase. When salvage therapy with imatinib was used in patients who did not respond or who responded poorly to induction with chemotherapy, it resulted in 9 (81.8%) out of 11 patients achieving a CHR. This clinical effect was associated with a greater reduction in BCR-ABL transcripts in the imatinib-treated patients than in the chemotherapy arm after 2 weeks of therapy (p=0.02). Following induction, all patients received imatinib (600 mg/day) combined with consolidation chemotherapy and the levels of BCR-ABL transcripts were comparable in the two arms at 8 weeks. Since patients received the same treatment in both arms after the induction phase, it was expected and observed that there was no difference in remission duration, disease-free survival or overall survival. However, it was observed that patients with complete molecular response and remaining in minimal residual disease had a better outcome in terms of both remission duration (p=0.01) and disease-free survival (p=0.02).
Three studies compared chemotherapy regimens integrating imatinib with historical data of chemotherapy alone.
In study AUS01 imatinib (400 mg/day on days 1 - 14 of each chemotherapy cycle during induction/consolidation, and 600 mg daily during maintenance treatment) was integrated into a HyperCVAD chemotherapy protocol, in Ph+ ALL patients with de novo disease (N=21), disease refractory to one cycle chemotherapy (N=5) or patients with a complete response after 1 cycle of chemotherapy (N=6). Results are summarized in Table 15.
 In study AJP01 imatinib (600 mg/day on days 8 - 63 of induction chemotherapy, and on days 1 - 28 of each chemotherapy cycle during consolidation and maintenance) was integrated into a chemotherapy regimen in 80 patients with de novo Ph+ ALL. Results are summarized in Table 16.
In study AJP01 imatinib (600 mg/day on days 8 - 63 of induction chemotherapy, and on days 1 - 28 of each chemotherapy cycle during consolidation and maintenance) was integrated into a chemotherapy regimen in 80 patients with de novo Ph+ ALL. Results are summarized in Table 16.
 Analysis of event-free survival and overall survival also indicated superiority of the imatinib-containing regimen (p < 0.0001 for both).
Analysis of event-free survival and overall survival also indicated superiority of the imatinib-containing regimen (p < 0.0001 for both).
In study AFR-09 imatinib (600 mg/day for two months during consolidation/salvage chemotherapy, and for two separate two-month periods during maintenance chemotherapy) was integrated into a chemotherapy regimen in 30 patients aged > 55 years with de novo Ph+ ALL. Results are summarized in Table 17.

Paediatric patients.
In study I2301, a total of 93 paediatric, adolescent and young adult patients (2 patients were < 2 years, 52 patients were from 2 to < 12 years, 32 patients were from 12 to < 18 years and 6 patients were from 18 to < 22 years) with Ph+ ALL were enrolled between 2002 and 2006 in an open-label, multicentre, sequential cohort, nonrandomised phase III trial, and were treated with imatinib (340 mg/m2/day) in combination with intensive chemotherapy after initial induction therapy. Imatinib was administered intermittently in cohorts 1-5, with increasing duration and earlier start of imatinib from cohort to cohort; cohort 1 receiving the lowest intensity and cohort 5 receiving the highest intensity of imatinib (longest duration in days with continuous daily imatinib dosing during the first chemotherapy treatment courses and intermittent schedule of imatinib 2 weeks every 4 weeks only during the last eight maintenance cycles). Continuous daily exposure to imatinib early in the course of treatment in combination with chemotherapy in cohort 5 patients (n=50) improved the 4-year event-free survival (EFS) compared to historical controls (n=120), who received standard chemotherapy without imatinib (69.6% vs. 31.6%, respectively). The estimated 4-year OS in cohort 5 patients was 83.6% compared to 44.8% in the historical controls (enrolled between 1988 and 1995).
Patients with (human leukocyte antigen (HLA)-matched related donors or 1 antigen mismatched (excluding HLA)-DR mismatch) related donors were eligible for HSCT on study after Consolidation block 2 (per protocol HSCT). At 16 to 24 weeks after per protocol HSCT, treatment with imatinib was resumed, initially at a lower dose of 230 mg/m2/day and increased to 340 mg/m2/day when no toxicities (≥ grade 3) were observed after 4 weeks of post-HSCT imatinib. The total duration of imatinib treatment post-HSCT was 24 weeks.
Relapsed/refractory Ph+ ALL.
A total of 429 patients with relapsed/refractory Ph+ALL or CML blast crisis were enrolled in three studies assessing the efficacy and safety of imatinib as monotherapy. Imatinib was given as single agent in 66 patients evaluable for efficacy and resulted in a haematological response rate of 33% (12% of which were complete) and a MCyR rate of 23%. The median time to progression for the overall population of 429 patients with relapsed/refractory Ph+ ALL ranged from 1.9 to 3.1 months, and median overall survival for the 409 evaluable patients ranged from 5 to 9 months.
Of the 429 patients described above, 146 were ≥ 55 years of age. The median time to progression and overall survival in this sub-population was similar to the overall population.
Clinical studies in myelodysplastic/myeloproliferative diseases (MDS/MPD) expressing imatinib-sensitive kinases. One open label, multinational, multicentre, phase II clinical trial (study B2225) was conducted testing imatinib in diverse populations of patients suffering from very rare and life-threatening diseases associated with ABL, KIT or PDGFR protein tyrosine kinases. This study included 7 patients with MDS/MPD out of a total of 185 patients treated, 45 of whom had haematological diseases and 140 a variety of solid tumours. These patients were treated with imatinib 400 mg daily, with provision to increase to 800 mg daily. The ages of the enrolled patients ranged from 20 to 86 years. A further 24 patients with MDS/MPD aged 2 to 79 years were reported in 12 published case reports and a clinical study. These patients also received imatinib at a dose of 400 mg daily with the exception of three patients who received lower doses. Of the total population of 31 patients treated for MDS/MPD, 14 (45%) achieved a complete haematological response and 9 (29%) a complete cytogenetic response (39% including major and partial responses). Of note, the malignancy carried a translocation, usually involving the chromosome t5q33 or t4q12, resulting in a PDGFR gene rearrangement in 14 evaluable patients. All of these responded haematologically (12 completely). Cytogenetic response was evaluated in 11 out of 14 patients, all of whom responded (9 patients completely). Only 2 (13%) out of the 16 patients without a translocation associated with PDGFR gene re-arrangement achieved a complete haematological response and one (6%) achieved a major cytogenetic response. A further patient with a PDGFR gene re-arrangement in molecular relapse after bone marrow transplant responded molecularly (RT-PCR negative for PDGFRβ fusion oncogene). Median duration of therapy was 12.9 months (0.8 - 26.7) in the 7 patients treated within study B2225 and ranged between 1 week and more than 18 months in responding patients in the published literature. Results are provided in Table 18.
 Clinical studies in aggressive systemic mastocytosis (ASM). Study B2225 also included 5 patients with ASM out of a total of 185 patients enrolled. The ASM patients were treated with imatinib 100 mg to 400 mg daily, with provision to increase the dose to 800 mg daily. The ages of these patients ranged from 49 to 74 years. A further 25 patients with ASM aged 26 to 85 years were reported in 10 published case reports and case series. These patients also received imatinib at doses of 100 mg to 400 mg daily. Of the total population of 30 patients treated for ASM, 10 (33%) achieved a complete haematological response and 9 (30%) a partial haematological response (63% overall response rate). Cytogenetic abnormalities were evaluated in 21 of the 30 patients treated. Eight out of these 21 patients had FIP1L1-PDGFRα fusion kinase. Patients with this cytogenetic abnormality are most likely to be males and to have eosinophilia associated with their systemic mast cell disease. Two patients showed a KIT mutation in the juxtamembrane region (one Phe522Cys and one K509I). Sixteen patients had unknown or no detected cytogenetic abnormality. Four patients showed a D816V mutation (the one responder had concomitant CML and SM). The majority of patients reported in the reviewed literature with the D816V c-KIT mutation were not considered sensitive to imatinib. Median duration of therapy was 13 months (range 1.4 to 22.3 months) in the 5 patients treated within study B2225 and ranged between 1 month and more than 30 months in responding patients in the published literature. Results are provided in Table 19.
Clinical studies in aggressive systemic mastocytosis (ASM). Study B2225 also included 5 patients with ASM out of a total of 185 patients enrolled. The ASM patients were treated with imatinib 100 mg to 400 mg daily, with provision to increase the dose to 800 mg daily. The ages of these patients ranged from 49 to 74 years. A further 25 patients with ASM aged 26 to 85 years were reported in 10 published case reports and case series. These patients also received imatinib at doses of 100 mg to 400 mg daily. Of the total population of 30 patients treated for ASM, 10 (33%) achieved a complete haematological response and 9 (30%) a partial haematological response (63% overall response rate). Cytogenetic abnormalities were evaluated in 21 of the 30 patients treated. Eight out of these 21 patients had FIP1L1-PDGFRα fusion kinase. Patients with this cytogenetic abnormality are most likely to be males and to have eosinophilia associated with their systemic mast cell disease. Two patients showed a KIT mutation in the juxtamembrane region (one Phe522Cys and one K509I). Sixteen patients had unknown or no detected cytogenetic abnormality. Four patients showed a D816V mutation (the one responder had concomitant CML and SM). The majority of patients reported in the reviewed literature with the D816V c-KIT mutation were not considered sensitive to imatinib. Median duration of therapy was 13 months (range 1.4 to 22.3 months) in the 5 patients treated within study B2225 and ranged between 1 month and more than 30 months in responding patients in the published literature. Results are provided in Table 19.
 Imatinib has not been shown to be effective in patients with less aggressive forms of systemic mastocytosis. Imatinib is not recommended for use in patients with cutaneous mastocytosis, indolent systemic mastocytosis (smoldering SM or isolated bone marrow mastocytosis), SM with an associated clonal haematological non-mast cell lineage disease, mast cell leukaemia, mast cell sarcoma or extracutaneous mastocytoma. In vitro, cell lines and patient-derived mast cells harbouring the KIT D816V mutation were resistant to imatinib and the effectiveness of imatinib in the treatment of patients with SM who have the D816V mutation remains controversial.
Imatinib has not been shown to be effective in patients with less aggressive forms of systemic mastocytosis. Imatinib is not recommended for use in patients with cutaneous mastocytosis, indolent systemic mastocytosis (smoldering SM or isolated bone marrow mastocytosis), SM with an associated clonal haematological non-mast cell lineage disease, mast cell leukaemia, mast cell sarcoma or extracutaneous mastocytoma. In vitro, cell lines and patient-derived mast cells harbouring the KIT D816V mutation were resistant to imatinib and the effectiveness of imatinib in the treatment of patients with SM who have the D816V mutation remains controversial.
Clinical studies in hypereosinophilic syndrome and/or chronic eosinophilic leukaemia (HES/CEL). Study B2225 also included 14 patients with HES/CEL out of a total of 185 patients enrolled. These patients were treated with 100 mg to 1,000 mg of imatinib daily. The ages of these patients ranged from 16 to 64 years. A further 162 patients with HES/CEL aged 11 to 78 years were reported in 35 published case reports and case series with a cut-off date of 15-Jan-06. These patients received imatinib at doses of 75 mg to 800 mg daily. Of the total population of 176 patients treated for HES/CEL, 107 (61%) achieved a complete haematological response and 16 (9%) a partial haematological response (70% overall response rate). Cytogenetic abnormalities were evaluated in 117 of the 176 patients treated. Out of these 117 patients, 61 were positive for FIP1L1-PDGFRα fusion kinase. All these FIP1L1-PDGFRα fusion kinase positive patients achieved a complete haematological response. The FIP1L1-PDGFRα fusion kinase was either negative or unknown in 115 patients, of which 62 (54%) achieved either a complete (N=46) or partial (N=16) haematological response. Results are provided in Table 20.
 Additionally, improvements in symptomatology and other organ dysfunction abnormalities were reported by the investigators in the case reports. Improvements were reported in cardiac, nervous, skin/subcutaneous tissue, respiratory/thoracic/mediastinal, musculoskeletal/ connective tissue/vascular, and gastrointestinal organ systems.
Additionally, improvements in symptomatology and other organ dysfunction abnormalities were reported by the investigators in the case reports. Improvements were reported in cardiac, nervous, skin/subcutaneous tissue, respiratory/thoracic/mediastinal, musculoskeletal/ connective tissue/vascular, and gastrointestinal organ systems.
Clinical studies in unresectable or metastatic gastrointestinal stromal tumours (GIST). Two open-label, randomised, multinational Phase III studies (SWOG, EORTC) were conducted in patients with unresectable or metastatic malignant gastrointestinal stromal tumours (GIST). The design of these two studies were similar allowing a predefined combined analysis of safety and efficacy. A total of 1,640 patients were enrolled into the two studies and randomized 1:1 to receive either 400 mg or 800 mg orally q.d. continuously until disease progression or unacceptable toxicity. Patients in the 400 mg q.d. treatment group who experienced disease progression were permitted to crossover to receive treatment with 800 mg q.d. The studies were designed to compare response rates, progression free survival and overall survival between the dose groups. Median age at patient entry was 60 (range 17 to 94, 25th -75th age percentile 50 to 69). Males comprised 58% of the patients enrolled. All patients had a pathologic diagnosis of CD117 positive unresectable and/or metastatic malignant GIST.
The primary objective of the EORTC study was to evaluate progression free survival (PFS) with a secondary objective to evaluate overall survival (OS) The primary objective of the SWOG trial was to evaluate OS with a secondary objective, PFS. A planned analysis of both OS and PFS from the combined datasets from these two studies was conducted. Neither study showed an advantage for the higher daily dose of 800 mg, so the data from both trials were pooled. No benefit of the higher dose was seen in OS or in Best Overall Tumour Response rates from the analysis of the pooled data, but a statistically significant increase in PFS was shown (Table 21).
 Median follow up for the combined studies was 37.5 months (25th - 75th percentile 19 to 46 months). There was a statistically significant improvement in PFS in the 800 mg treatment group (23.2 months [95% CI, 20.8 to 24.9]) compared to the 400 mg treatment group (18.9 months [95% CI, 17.4 to 21.2]) (p=0.03). However, there were no observed differences in overall survival between the treatment groups (p=0.98). The estimated overall PFS for all 1640 patients in these Phase III studies was 21 months [95% CI 19.4 to 22.5] and the estimated OS of 48.8 months [95% CI 46.3 to 51.6]. 5.1% of patients achieved a confirmed complete response and 47.5% achieved a partial response. Treatment at either dose level was generally well tolerated and overall, 5.4% of patients withdrew due to toxicity.
Median follow up for the combined studies was 37.5 months (25th - 75th percentile 19 to 46 months). There was a statistically significant improvement in PFS in the 800 mg treatment group (23.2 months [95% CI, 20.8 to 24.9]) compared to the 400 mg treatment group (18.9 months [95% CI, 17.4 to 21.2]) (p=0.03). However, there were no observed differences in overall survival between the treatment groups (p=0.98). The estimated overall PFS for all 1640 patients in these Phase III studies was 21 months [95% CI 19.4 to 22.5] and the estimated OS of 48.8 months [95% CI 46.3 to 51.6]. 5.1% of patients achieved a confirmed complete response and 47.5% achieved a partial response. Treatment at either dose level was generally well tolerated and overall, 5.4% of patients withdrew due to toxicity.
Patients who crossed over following disease progression from the 400 mg/day treatment group to the 800 mg/day treatment (N=347) had a 3.4 month median and 7.7 month mean exposure to imatinib following crossover. Overall survival of patients following crossover was 14.3 months [95% CI 12.2 to 16.7] and 19.3% of these patients were still alive at 48 months.
One phase II, open-label, randomised multinational study was conducted in patients with unresectable or metastatic malignant gastrointestinal stromal tumours (GIST). In this study 147 patients were enrolled and randomised to receive either 400 mg or 600 mg imatinib orally daily for up to 36 months. These patients ranged in age from 18 to 83 years old and had a pathologic diagnosis of KIT-positive malignant GIST that was unresectable and/or metastatic.
The primary evidence of efficacy was based on objective response rates. Tumours were required to be measurable in at least one site of disease and response characterisation was based on Southwestern Oncology Group (SWOG) criteria. In this study, 83% of the patients achieved either a complete response, partial response or stable disease. Results are provided in Table 22.
 A statistically significant difference in response rates between the two dose groups was not demonstrated. A significant number of patients who had stable disease at the time of the interim analysis achieved a partial response with longer treatment (median follow-up 31 months). Median time to response was 13 weeks (95% C.I. 12 to 23). Median time to treatment failure in responders was 122 weeks (95% C.I. 106 to 147), while in the overall study population it was 84 weeks (95% C.I. 71 to 109). The median overall survival has not been reached. The Kaplan-Meier estimate for survival after 36-month follow-up is 68% [Figure 3]. Additionally, there is no difference in survival between patients achieving stable disease and partial response [Figure 4].
A statistically significant difference in response rates between the two dose groups was not demonstrated. A significant number of patients who had stable disease at the time of the interim analysis achieved a partial response with longer treatment (median follow-up 31 months). Median time to response was 13 weeks (95% C.I. 12 to 23). Median time to treatment failure in responders was 122 weeks (95% C.I. 106 to 147), while in the overall study population it was 84 weeks (95% C.I. 71 to 109). The median overall survival has not been reached. The Kaplan-Meier estimate for survival after 36-month follow-up is 68% [Figure 3]. Additionally, there is no difference in survival between patients achieving stable disease and partial response [Figure 4].

 Clinical studies in adjuvant GIST. In the adjuvant setting, imatinib was investigated in a multicentre, double-blind, long-term, placebo controlled phase III study (Z9001) involving 713 patients. The ages of these patients ranged from 18 to 91 years. Patients were included who had a histologic diagnosis of primary GIST expressing KIT protein by immunochemistry and a tumour size ≥ 3 cm in maximum dimension, with complete gross resection of primary GIST within 14 to 70 days prior to registration. After resection of primary GIST, patients were randomized to one of the two arms: imatinib at 400 mg/day or matching placebo for one year.
Clinical studies in adjuvant GIST. In the adjuvant setting, imatinib was investigated in a multicentre, double-blind, long-term, placebo controlled phase III study (Z9001) involving 713 patients. The ages of these patients ranged from 18 to 91 years. Patients were included who had a histologic diagnosis of primary GIST expressing KIT protein by immunochemistry and a tumour size ≥ 3 cm in maximum dimension, with complete gross resection of primary GIST within 14 to 70 days prior to registration. After resection of primary GIST, patients were randomized to one of the two arms: imatinib at 400 mg/day or matching placebo for one year.
The primary endpoint of the study was recurrence free survival (RFS) defined as the time from date of randomization to the date of recurrence or death from any cause.
In the planned interim analysis at a median follow-up of 15 months in patients without an RFS event, imatinib significantly prolonged RFS compared to placebo (hazard ratio 0.40, 95% CI [0.26, 0.61], p < 0.0001). After the interim analysis of RFS, 72 of the 354 patients randomized to placebo crossed over to one year of imatinib. In an updated analysis of 50 months median follow-up in patients without an event, the hazard ratio for RFS remained significant at 0.72, 95% CI: [0.53, 0.97]. At 61 months median survival follow-up (measured in patients still alive), the hazard ratio for overall survival (OS) was not significant but favoured imatinib: 0.82, 95% CI: [0.49, 1.37]. Death due to any cause was reported in 26 patients on imatinib (11 due to GIST) and 33 patients on placebo (18 due to GIST). An OS analysis censoring placebo patients eligible for cross-over to imatinib was also not significant but supportive of imatinib: HR=0.75; 95% CI: [0.44, 1.26].
A second open label phase III study (SSG XVIII/AIO) compared 400 mg/day imatinib 12 months treatment vs. 36 months treatment in patients after surgical resection of KIT-positive GIST and one of the following: tumour diameter > 5 cm and mitotic count > 5/50 high power fields (HPF); or tumour diameter > 10 cm and any mitotic count or tumour of any size with mitotic count >10/50 HPF or tumours ruptured into the peritoneal cavity. There were a total of 397 patients consented and randomized to the study (199 patients on 12 month arm and 198 patients on 36 month arm), median age was 61 years (range 22 to 84 years). The median time of follow-up was 54 months (from date of randomization to data cut-off), with a total of 83 months between the first patient randomized and the cut-off date.
The primary endpoint of the study was recurrence free survival (RFS) defined as the time from date of randomization to the date of recurrence or death from any cause.
Thirty-six (36) months of imatinib treatment significantly prolonged RFS compared to 12 months of imatinib treatment (with overall Hazard Ratio (HR) = 0.46 [0.32, 0.65], p < 0.0001 and a HR of 0.42 [0.28, 0.61] beyond month 12) (Table 23 and Figure 5). There were 84 (42%) and 50 (25%) total RFS events for the 12-months and 36 months arms respectively.
In addition, thirty-six (36) months of imatinib treatment significantly prolonged overall survival (OS) compared to 12 months of imatinib treatment (HR=0.45 [0.22, 0.89], p=0.0187) (Table 23 and Figure 6). The total number of deaths were 25 for the 12-months treatment arm and 12 for the 36-months treatment arm. Three randomized patients (one from 12-month group and two from the 36-month group) did not give informed consent and were excluded from analysis.


 Clinical studies in dermatofibrosarcoma protuberans (DFSP). Study B2225 (see Section 5.1 Pharmacodynamic Properties, Clinical studies in myelodysplastic/myeloproliferative diseases (MDS/MPD) expressing imatinib-sensitive kinases.) also included 12 patients with DFSP out of a total of 185 patients. The primary evidence of efficacy for patients in the solid tumour group (N=140) was based on objective response rates. The solid tumour population was treated with imatinib 800 mg daily. The age of the DFSP patients ranged from 23 to 75 years; DFSP was metastatic, locally recurrent following initial resective surgery and not considered amenable to further resective surgery at the time of study entry. A further 6 DFSP patients treated with imatinib were reported in 5 published case reports, their ages ranging from 18 months to 49 years. The total population treated for DFSP comprises 18 patients, 8 of them with metastatic disease. The adult patients reported in the published literature were treated with either 400 mg (4 cases) or 800 mg (1 case) imatinib daily. The paediatric patient received 400 mg/m2/daily, subsequently increased to 520 mg/m2/daily. Responses to treatment are described in Table 24.
Clinical studies in dermatofibrosarcoma protuberans (DFSP). Study B2225 (see Section 5.1 Pharmacodynamic Properties, Clinical studies in myelodysplastic/myeloproliferative diseases (MDS/MPD) expressing imatinib-sensitive kinases.) also included 12 patients with DFSP out of a total of 185 patients. The primary evidence of efficacy for patients in the solid tumour group (N=140) was based on objective response rates. The solid tumour population was treated with imatinib 800 mg daily. The age of the DFSP patients ranged from 23 to 75 years; DFSP was metastatic, locally recurrent following initial resective surgery and not considered amenable to further resective surgery at the time of study entry. A further 6 DFSP patients treated with imatinib were reported in 5 published case reports, their ages ranging from 18 months to 49 years. The total population treated for DFSP comprises 18 patients, 8 of them with metastatic disease. The adult patients reported in the published literature were treated with either 400 mg (4 cases) or 800 mg (1 case) imatinib daily. The paediatric patient received 400 mg/m2/daily, subsequently increased to 520 mg/m2/daily. Responses to treatment are described in Table 24.
 Twelve of these 18 patients either achieved a complete response (7 patients) or were made disease free by surgery after a partial response (5 patients, including one child) for a total complete response rate of 67%. A further 3 patients achieved a partial response, for an overall response rate of 83%. Of the 8 patients with metastatic disease, five responded (62%), three of them completely (37%). The median duration of therapy in study B2225 was 6.2 months, with a maximum duration of 24.3 months, while in the published literature it ranged between 4 weeks and more than 20 months.
Twelve of these 18 patients either achieved a complete response (7 patients) or were made disease free by surgery after a partial response (5 patients, including one child) for a total complete response rate of 67%. A further 3 patients achieved a partial response, for an overall response rate of 83%. Of the 8 patients with metastatic disease, five responded (62%), three of them completely (37%). The median duration of therapy in study B2225 was 6.2 months, with a maximum duration of 24.3 months, while in the published literature it ranged between 4 weeks and more than 20 months.
The effectiveness of imatinib is based on overall haematological and cytogenetic response rates and progression-free survival in CML, on haematological and cytogenetic response rates in Ph+ ALL, MDS/MPD, on haematological response rates in HES/CEL, SM, and progression-free survival in unresectable and/or metastatic and objective response rates in GIST, on recurrence free survival in adjuvant GIST, and on objective response rates in DFSP. There are no controlled trials demonstrating increased survival.
5.2 Pharmacokinetic Properties
The pharmacokinetics of imatinib have been evaluated over a dosage range of 25 to 1000 mg. Plasma pharmacokinetic profiles were analysed on day 1 and on either day 7 or day 28, by which time plasma concentrations had reached steady state.
Absorption.
Imatinib is well absorbed after oral administration, with maximum plasma concentrations (Cmax) being reached approximately 2 hours after dosing. When given with a high fat meal, the rate of absorption of imatinib was minimally reduced (11% decrease in Cmax and prolongation of tmax by 1.5 h), with a small reduction in AUC (7.4%) compared to fasting conditions.
The increase in AUC was linear and dose proportional in the range of 25-1000 mg imatinib after oral administration. There was no change in the kinetics of imatinib on repeated dosing, and accumulation was 1.5-2.5-fold at steady state when dosed once daily. Plasma concentrations of imatinib and its main metabolite showed significant inter-subject variability.
Distribution.
At clinically relevant concentrations of imatinib, binding to plasma proteins was approximately 95% on the basis of in vitro experiments, mostly to albumin and alpha-acid-glycoprotein, with little binding to lipoprotein. The volume of distribution is about 435 L.
Metabolism.
Imatinib is cleared from plasma predominantly by metabolism and CYP 3A4 is the main enzyme responsible. In healthy volunteers, clearance is approximately 14 L/hr and the drug has a half-life of approximately 18 hours, suggesting that once-daily dosing is appropriate.
The main circulating metabolite in humans is the N-demethylated piperazine derivative, which shows similar in vitro potency as imatinib. The plasma AUC for this metabolite was found to be only 16% of the AUC for imatinib. Its half-life was approximately 40 hours. The plasma protein binding of the N-demethylated metabolite is similar to that of imatinib.
Imatinib competitively inhibits CYP2C9, CYP2D6 and CYP3A4/5, with Ki values indicating that CYP2D6 and CYP3A4-dependent metabolism of concomitantly administered drugs may be reduced (see Section 4.5 Interactions with Other Medicines and Other Forms of Interactions).
Excretion.
Based on the recovery of compound(s) after an oral 14C-labelled dose of imatinib, approximately 81% of the dose was eliminated within 7 days in faeces (68% of dose) and urine (13% of dose). Unchanged imatinib accounted for 25% of the dose (5% urine, 20% faeces), the remainder being metabolites.
Pharmacokinetics in special patient groups.
Based on population pharmacokinetic analysis, there was a small effect of age on the volume of distribution (12% increase in patients > 65 years old). This change is not thought to be clinically significant. No significant age related pharmacokinetic differences have been observed in adult patients in clinical trials which included over 20% of patients age 65 and older.
The effect of body weight on the clearance of imatinib is such that for a 50 year old patient weighing 50 kg the mean clearance is expected to be 8 L/h, while for a 50 year old patient weighing 100 kg the clearance will rise to 14 L/h. These changes are not considered sufficient to warrant dose adjustment based on kg bodyweight. There is no effect of gender on the kinetics of imatinib.
Pharmacokinetics in children.
As in adult patients, imatinib was rapidly absorbed after oral administration in children in both Phase I and II studies, with a Cmax of 2-4 hours. Apparent oral clearance was also similar (mean 11.0 L/h/m2 in children vs. 10.0 L/h/m2 in adults) as was half-life (mean 14.8 h in children vs. 17.1 h in adults). Dosing in children at both 260 mg/m2 and 340 mg/m2 achieved an AUC similar to a 400 mg and 600 mg dose, respectively, in adults. After repeated once daily dosing at 260 mg/m2 and 340 mg/m2, drug accumulation was 1.5 and 2.2-fold respectively, on comparing AUC0-24h on days 1 and 8. Mean imatinib AUC did not increase proportionally with increasing dose.
Based on pooled population pharmacokinetic analysis in paediatric patients with haematological disorders (CML, Ph+ALL, or other haematological disorders treated with imatinib), clearance of imatinib increases with increasing body surface area (BSA). After correcting for the BSA effect, other demographics such as age, body weight and body mass index did not have clinically significant effects on the exposure of imatinib. The analysis confirmed that exposure of imatinib in paediatric patients receiving 260 mg/m2 once daily (not exceeding 400 mg once daily) or 340 mg/m2 once daily (not exceeding 600 mg once daily) were similar to those in adult patients who received imatinib 400 mg or 600 mg once daily. No pharmacokinetic data have been obtained in children < 2 years of age.
Pharmacokinetics in patients with impaired renal or hepatic function.
In a study of patients with varying degrees of hepatic dysfunction (mild, moderate and severe - see Table 25 for classification of these terms), the mean exposure to imatinib (dose normalised AUC) showed similar exposure between patients with mild and moderate impairment, but an approximately 45% higher exposure in patients with severe impairment. In this study, 500 mg daily was safely used in patients with mild liver impairment and 300 mg daily was used in other patients. Although only a 300 mg daily dose was used in patients with moderate and severe liver impairment, pharmacokinetic analysis projects that 400 mg can be used safely in patients with moderate liver impairment, and a dose of 300 mg can be used for patients with severe liver impairment (see Section 4.4 Special Warnings and Precautions for Use; Section 4.8 Adverse Effects (Undesirable Effects); Section 4.2 Dose and Method of Administration). Imatinib Sandoz should be used with caution in patients with liver impairment (see Section 4.4 Special Warnings and Precautions for Use).
 Imatinib and its metabolites are not excreted via the kidney to a significant extent. In a study of patients with varying degrees of renal dysfunction (mild, moderate and severe - see Table 26 for renal function classification), the mean exposure to imatinib (dose normalized AUC) increased 1.5- to 2-fold compared to patients with normal renal function, which corresponded to an elevated plasma level of AGP, a protein to which imatinib binds strongly. No correlation between imatinib exposure and the severity of renal deficiency was observed. In this study, 800 mg daily was safely used in patients with mild renal dysfunction and 600 mg daily was used in moderate renal dysfunction. The 800 mg dose was not tested in patients with moderate renal dysfunction due to the limited number of patients enrolled. Similarly, only 2 patients with severe renal dysfunction were enrolled at the low (100 mg) dose, and no higher doses were tested. No patients on haemodialysis were enrolled in the study. Literature data showed that a daily dose of 400 mg was well tolerated in a patient with end stage renal disease on haemodialysis. The PK plasma exposure in this patient fell within the range of values of imatinib and its metabolite CGP74588 observed in patients with normal renal function. Dialysis was not found to intervene with the plasma kinetics of imatinib. Since renal excretion represents a minor elimination pathway for imatinib, patients with severe renal insufficiency and on dialysis could receive treatment at the 400 mg starting dose. However, in these patients caution is recommended. The dose can be reduced if not tolerated, or increased for lack of efficacy (see Section 4.4 Special Warnings and Precautions for Use; Section 4.2 Dose and Method of Administration).
Imatinib and its metabolites are not excreted via the kidney to a significant extent. In a study of patients with varying degrees of renal dysfunction (mild, moderate and severe - see Table 26 for renal function classification), the mean exposure to imatinib (dose normalized AUC) increased 1.5- to 2-fold compared to patients with normal renal function, which corresponded to an elevated plasma level of AGP, a protein to which imatinib binds strongly. No correlation between imatinib exposure and the severity of renal deficiency was observed. In this study, 800 mg daily was safely used in patients with mild renal dysfunction and 600 mg daily was used in moderate renal dysfunction. The 800 mg dose was not tested in patients with moderate renal dysfunction due to the limited number of patients enrolled. Similarly, only 2 patients with severe renal dysfunction were enrolled at the low (100 mg) dose, and no higher doses were tested. No patients on haemodialysis were enrolled in the study. Literature data showed that a daily dose of 400 mg was well tolerated in a patient with end stage renal disease on haemodialysis. The PK plasma exposure in this patient fell within the range of values of imatinib and its metabolite CGP74588 observed in patients with normal renal function. Dialysis was not found to intervene with the plasma kinetics of imatinib. Since renal excretion represents a minor elimination pathway for imatinib, patients with severe renal insufficiency and on dialysis could receive treatment at the 400 mg starting dose. However, in these patients caution is recommended. The dose can be reduced if not tolerated, or increased for lack of efficacy (see Section 4.4 Special Warnings and Precautions for Use; Section 4.2 Dose and Method of Administration).

5.3 Preclinical Safety Data
In mice in vivo, imatinib inhibits tumour growth of BCR-ABL transfected murine myeloid cells as well as BCR-ABL positive leukaemia cell lines derived from CML patients.
Toxicities for long term use.
Liver toxicity was observed in the 3 species tested in repeated-dose studies (rats, dogs and cynomolgus monkeys), and was most severe in dogs, in which findings included elevated liver enzymes, hepatocellular necrosis, and bile duct necrosis and hyperplasia.
Renal toxicity was observed in rats and monkeys, being most marked in the latter, with increases in blood urea nitrogen (BUN) and creatinine being observed in several animals, and with findings including renal tubular dilatation, mineralisation and nephrosis. Lymphoid tissue atrophy was observed in many of the repeated-dose animal studies and lymphopenia was sometimes observed in animals (as in humans). In a 39-week monkey study, treatment with imatinib apparently resulted in worsening of malarial infections which were normally suppressed in these animals (see Section 4.4 Special Warnings and Precautions for Use).
Genotoxicity.
Positive genotoxic effects were observed for imatinib in an in vitro mammalian cell assay (Chinese hamster ovary) for clastogenicity (chromosome aberrations) in the presence of metabolic activation, but imatinib was negative in an in vivo rat micronucleus assay. Imatinib was not genotoxic when tested in assays for gene mutations (in vitro bacterial cell assay and an in vitro mouse lymphoma assay). Two intermediates of the manufacturing process, which are also present in the final product, are positive for mutagenesis in the Ames assay. One of these intermediates was also positive in the mouse lymphoma assay.
Carcinogenicity.
In a 2-year rat carcinogenicity study administration of imatinib at 15, 30 and 60 mg/kg/day resulted in neoplastic changes in the kidneys, urinary bladder, urethra, preputial and clitoral gland, small intestine, parathyroid glands, adrenal glands and non-glandular stomach. The no observed effect levels (NOEL) for the various target organs with neoplastic lesions were established as follows: 30 mg/kg/day for the kidneys, urinary bladder, urethra, small intestine, parathyroid glands, adrenal glands and non-glandular stomach, and 15 mg/kg/day for the preputial and clitoral glands.
The 30 mg/kg/day dose in rats is approximately equivalent to the human daily exposure (based on plasma AUC) at 800 mg/day and 2 times the daily exposure in children (based on AUC) at 340 mg/m2.
These findings are potentially relevant for patients receiving long-term imatinib therapy. However, an analysis of the safety data from clinical trials and spontaneous adverse event reports did not provide evidence of an increase in overall incidence of malignancies, or the incidence of bladder, kidney or prostate tumours in patients treated with imatinib compared to that of the general population.6 Pharmaceutical Particulars
6.1 List of Excipients
Tablets.
Cellulose-microcrystalline, crospovidone, hypromellose, silica colloidal anhydrous, magnesium stearate, iron oxide yellow CI 77492, iron oxide red CI77491, macrogol 4000, talc.
6.2 Incompatibilities
Incompatibilities were either not assessed or not identified as part of the registration of this medicine.
6.3 Shelf Life
In Australia, information on the shelf life can be found on the public summary of the Australian Register of Therapeutic Goods (ARTG). The expiry date can be found on the packaging.
6.4 Special Precautions for Storage
Imatinib Sandoz 100 mg film-coated tablet.
Store in the original package below 30°C and protect from moisture.
Imatinib Sandoz 400 mg film-coated tablet (PVC/PE/PVDC/Al blister).
Store in the original package below 25°C and protect from moisture.
Imatinib Sandoz 400 mg film-coated tablet (PA/Al/PVC blister).
Store in the original package below 30°C and protect from moisture.
Keep out of the reach of children.
6.5 Nature and Contents of Container
Imatinib Sandoz 100 mg film-coated tablet.
Blister packs, PVC/PE/PVDC/Al containing 56, 60, 180 tablets.
Imatinib Sandoz 400 mg film-coated tablet.
Blister packs, PVC/PE/PVDC/Al and PA/Al/PVC containing 28, 30 tablets.
Not all presentations/pack sizes are available in Australia.
6.6 Special Precautions for Disposal
In Australia, any unused medicine or waste material should be disposed of in accordance with local requirements.
6.7 Physicochemical Properties
Imatinib mesilate is a white to slightly yellowish powder. It is freely soluble in water and aqueous buffers ≤ pH 5.5 and less soluble in more neutral/alkaline aqueous buffers. In non-aqueous solvents, the compound is soluble in dimethyl sulfoxide, methanol and ethanol, but is insoluble in n-octanol, acetone and acetonitrile.
Chemical structure.
 Chemical name: 4-[(4-methyl-1-piperazinyl) methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl] amino]-phenyl] benzamide methanesulfonate.
Chemical name: 4-[(4-methyl-1-piperazinyl) methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl] amino]-phenyl] benzamide methanesulfonate.
Molecular formula: C29H31N7O.CH4SO3.
Molecular weight: 493.6 (free base) + 96.1 (mesilate) = 589.7.
CAS number.
152459-95-5 (free base); 220127-57-1 (mesilate).7 Medicine Schedule (Poisons Standard)
Prescription Only Medicine (Schedule 4).
Summary Table of Changes


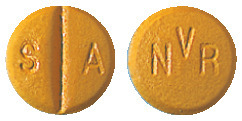
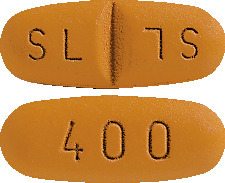




 The adjuvant trial SSG XVIII/AIO compared 1 year and 3 years imatinib treatment. Table 6 shows adverse events, regardless of relationship to study drug, that were reported in at least 5% of patients treated with imatinib. There was a higher incidence of severe (Grade 3-4) and serious adverse events in the 3-year group than the 1-year group, but this was expected because of the longer duration of treatment. Discontinuations due to adverse events were also greater in the 3-year group (13.6%) than the 1-year group (7.7%). Adverse events were generally consistent with the known safety profile of imatinib. There was insufficient data to assess long-term safety.
The adjuvant trial SSG XVIII/AIO compared 1 year and 3 years imatinib treatment. Table 6 shows adverse events, regardless of relationship to study drug, that were reported in at least 5% of patients treated with imatinib. There was a higher incidence of severe (Grade 3-4) and serious adverse events in the 3-year group than the 1-year group, but this was expected because of the longer duration of treatment. Discontinuations due to adverse events were also greater in the 3-year group (13.6%) than the 1-year group (7.7%). Adverse events were generally consistent with the known safety profile of imatinib. There was insufficient data to assess long-term safety. Adverse events with imatinib observed in trials in Ph+ ALL, MDS/MPD, SM, HES/CEL and DFSP were generally consistent with those seen in CML and GIST studies.
Adverse events with imatinib observed in trials in Ph+ ALL, MDS/MPD, SM, HES/CEL and DFSP were generally consistent with those seen in CML and GIST studies.


 Laboratory abnormalities with imatinib observed in trials in Ph+ ALL, MDS/MPD, SM, HES/CEL and DFSP were generally consistent with those seen in CML and GIST studies.
Laboratory abnormalities with imatinib observed in trials in Ph+ ALL, MDS/MPD, SM, HES/CEL and DFSP were generally consistent with those seen in CML and GIST studies. Rates of complete haematological response, major cytogenetic response and complete cytogenetic
response on first-line treatment were estimated using the Kaplan-Meier approach, for which non-responses were censored at the date of last examination. Using this approach the estimated cumulative response rates for first-line treatment with imatinib are shown in Table 12.
Rates of complete haematological response, major cytogenetic response and complete cytogenetic
response on first-line treatment were estimated using the Kaplan-Meier approach, for which non-responses were censored at the date of last examination. Using this approach the estimated cumulative response rates for first-line treatment with imatinib are shown in Table 12. For analysis of long-term outcomes patients randomised to receive imatinib were compared with patients randomised to receive IFN. Patients who crossed over prior to progression were not censored at the time of crossover, and events that occurred in these patients following crossover were attributed to the original randomised treatment.
For analysis of long-term outcomes patients randomised to receive imatinib were compared with patients randomised to receive IFN. Patients who crossed over prior to progression were not censored at the time of crossover, and events that occurred in these patients following crossover were attributed to the original randomised treatment.
 A total of 71 (12.8%) and 85 (15.4%) patients died in the imatinib and IFN+Ara-C groups, respectively. At 84 months the estimated overall survival is 86.4% (83, 90) vs. 83.3% (80, 87) in the randomised imatinib and the IFN+Ara-C groups, respectively (p=0.073, log-rank test). This time-to-event endpoint is strongly affected by the high crossover rate from IFN+Ara-C to imatinib. Additionally, a greater number of patients received bone marrow transplant (BMT) after discontinuation of study treatment in the IFN+Ara-C group (n=66, 38 after crossover to imatinib) compared with the imatinib group (n=50, 8 after crossover to IFN) at the 84 month update. When censoring the 48 deaths that occurred after BMT, the 84-months survival rates were 89.6 vs 88.1 (p=0.200, log-rank test). Only 31 deaths (before BMT) of the imatinib patients (5.6%) were attributed to CML, compared to 40 of the IFN+Ara-C patients (7.2%). When only considering these CML-related deaths and censoring any deaths after BMT or due to other reasons, the estimated 84-months survival rates were 93.6% vs. 91.1% (p=0.1, log rank test). The effect of imatinib treatment on survival in chronic phase, newly diagnosed CML has been further examined in a retrospective analysis of the above reported imatinib data with the primary data from another Phase III study using IFN+Ara-C (n=325) in an identical regimen. In this publication, the superiority of imatinib over IFN+Ara-C in overall survival was demonstrated (p < 0.001); within 42 months, 47 (8.5%) imatinib patients and 63 (19.4%) IFN+Ara-C patients had died.
A total of 71 (12.8%) and 85 (15.4%) patients died in the imatinib and IFN+Ara-C groups, respectively. At 84 months the estimated overall survival is 86.4% (83, 90) vs. 83.3% (80, 87) in the randomised imatinib and the IFN+Ara-C groups, respectively (p=0.073, log-rank test). This time-to-event endpoint is strongly affected by the high crossover rate from IFN+Ara-C to imatinib. Additionally, a greater number of patients received bone marrow transplant (BMT) after discontinuation of study treatment in the IFN+Ara-C group (n=66, 38 after crossover to imatinib) compared with the imatinib group (n=50, 8 after crossover to IFN) at the 84 month update. When censoring the 48 deaths that occurred after BMT, the 84-months survival rates were 89.6 vs 88.1 (p=0.200, log-rank test). Only 31 deaths (before BMT) of the imatinib patients (5.6%) were attributed to CML, compared to 40 of the IFN+Ara-C patients (7.2%). When only considering these CML-related deaths and censoring any deaths after BMT or due to other reasons, the estimated 84-months survival rates were 93.6% vs. 91.1% (p=0.1, log rank test). The effect of imatinib treatment on survival in chronic phase, newly diagnosed CML has been further examined in a retrospective analysis of the above reported imatinib data with the primary data from another Phase III study using IFN+Ara-C (n=325) in an identical regimen. In this publication, the superiority of imatinib over IFN+Ara-C in overall survival was demonstrated (p < 0.001); within 42 months, 47 (8.5%) imatinib patients and 63 (19.4%) IFN+Ara-C patients had died.
 Efficacy results were similar in men and women and in patients younger and older than age 65. Responses were seen in black patients, but there were too few black patients to allow a quantitative comparison.
Efficacy results were similar in men and women and in patients younger and older than age 65. Responses were seen in black patients, but there were too few black patients to allow a quantitative comparison. In study AJP01 imatinib (600 mg/day on days 8 - 63 of induction chemotherapy, and on days 1 - 28 of each chemotherapy cycle during consolidation and maintenance) was integrated into a chemotherapy regimen in 80 patients with de novo Ph+ ALL. Results are summarized in Table 16.
In study AJP01 imatinib (600 mg/day on days 8 - 63 of induction chemotherapy, and on days 1 - 28 of each chemotherapy cycle during consolidation and maintenance) was integrated into a chemotherapy regimen in 80 patients with de novo Ph+ ALL. Results are summarized in Table 16. Analysis of event-free survival and overall survival also indicated superiority of the imatinib-containing regimen (p < 0.0001 for both).
Analysis of event-free survival and overall survival also indicated superiority of the imatinib-containing regimen (p < 0.0001 for both).

 Imatinib has not been shown to be effective in patients with less aggressive forms of systemic mastocytosis. Imatinib is not recommended for use in patients with cutaneous mastocytosis, indolent systemic mastocytosis (smoldering SM or isolated bone marrow mastocytosis), SM with an associated clonal haematological non-mast cell lineage disease, mast cell leukaemia, mast cell sarcoma or extracutaneous mastocytoma. In vitro, cell lines and patient-derived mast cells harbouring the KIT D816V mutation were resistant to imatinib and the effectiveness of imatinib in the treatment of patients with SM who have the D816V mutation remains controversial.
Imatinib has not been shown to be effective in patients with less aggressive forms of systemic mastocytosis. Imatinib is not recommended for use in patients with cutaneous mastocytosis, indolent systemic mastocytosis (smoldering SM or isolated bone marrow mastocytosis), SM with an associated clonal haematological non-mast cell lineage disease, mast cell leukaemia, mast cell sarcoma or extracutaneous mastocytoma. In vitro, cell lines and patient-derived mast cells harbouring the KIT D816V mutation were resistant to imatinib and the effectiveness of imatinib in the treatment of patients with SM who have the D816V mutation remains controversial. Additionally, improvements in symptomatology and other organ dysfunction abnormalities were reported by the investigators in the case reports. Improvements were reported in cardiac, nervous, skin/subcutaneous tissue, respiratory/thoracic/mediastinal, musculoskeletal/ connective tissue/vascular, and gastrointestinal organ systems.
Additionally, improvements in symptomatology and other organ dysfunction abnormalities were reported by the investigators in the case reports. Improvements were reported in cardiac, nervous, skin/subcutaneous tissue, respiratory/thoracic/mediastinal, musculoskeletal/ connective tissue/vascular, and gastrointestinal organ systems. Median follow up for the combined studies was 37.5 months (25th - 75th percentile 19 to 46 months). There was a statistically significant improvement in PFS in the 800 mg treatment group (23.2 months [95% CI, 20.8 to 24.9]) compared to the 400 mg treatment group (18.9 months [95% CI, 17.4 to 21.2]) (p=0.03). However, there were no observed differences in overall survival between the treatment groups (p=0.98). The estimated overall PFS for all 1640 patients in these Phase III studies was 21 months [95% CI 19.4 to 22.5] and the estimated OS of 48.8 months [95% CI 46.3 to 51.6]. 5.1% of patients achieved a confirmed complete response and 47.5% achieved a partial response. Treatment at either dose level was generally well tolerated and overall, 5.4% of patients withdrew due to toxicity.
Median follow up for the combined studies was 37.5 months (25th - 75th percentile 19 to 46 months). There was a statistically significant improvement in PFS in the 800 mg treatment group (23.2 months [95% CI, 20.8 to 24.9]) compared to the 400 mg treatment group (18.9 months [95% CI, 17.4 to 21.2]) (p=0.03). However, there were no observed differences in overall survival between the treatment groups (p=0.98). The estimated overall PFS for all 1640 patients in these Phase III studies was 21 months [95% CI 19.4 to 22.5] and the estimated OS of 48.8 months [95% CI 46.3 to 51.6]. 5.1% of patients achieved a confirmed complete response and 47.5% achieved a partial response. Treatment at either dose level was generally well tolerated and overall, 5.4% of patients withdrew due to toxicity. A statistically significant difference in response rates between the two dose groups was not demonstrated. A significant number of patients who had stable disease at the time of the interim analysis achieved a partial response with longer treatment (median follow-up 31 months). Median time to response was 13 weeks (95% C.I. 12 to 23). Median time to treatment failure in responders was 122 weeks (95% C.I. 106 to 147), while in the overall study population it was 84 weeks (95% C.I. 71 to 109). The median overall survival has not been reached. The Kaplan-Meier estimate for survival after 36-month follow-up is 68% [Figure 3]. Additionally, there is no difference in survival between patients achieving stable disease and partial response [Figure 4].
A statistically significant difference in response rates between the two dose groups was not demonstrated. A significant number of patients who had stable disease at the time of the interim analysis achieved a partial response with longer treatment (median follow-up 31 months). Median time to response was 13 weeks (95% C.I. 12 to 23). Median time to treatment failure in responders was 122 weeks (95% C.I. 106 to 147), while in the overall study population it was 84 weeks (95% C.I. 71 to 109). The median overall survival has not been reached. The Kaplan-Meier estimate for survival after 36-month follow-up is 68% [Figure 3]. Additionally, there is no difference in survival between patients achieving stable disease and partial response [Figure 4].




 Twelve of these 18 patients either achieved a complete response (7 patients) or were made disease free by surgery after a partial response (5 patients, including one child) for a total complete response rate of 67%. A further 3 patients achieved a partial response, for an overall response rate of 83%. Of the 8 patients with metastatic disease, five responded (62%), three of them completely (37%). The median duration of therapy in study B2225 was 6.2 months, with a maximum duration of 24.3 months, while in the published literature it ranged between 4 weeks and more than 20 months.
Twelve of these 18 patients either achieved a complete response (7 patients) or were made disease free by surgery after a partial response (5 patients, including one child) for a total complete response rate of 67%. A further 3 patients achieved a partial response, for an overall response rate of 83%. Of the 8 patients with metastatic disease, five responded (62%), three of them completely (37%). The median duration of therapy in study B2225 was 6.2 months, with a maximum duration of 24.3 months, while in the published literature it ranged between 4 weeks and more than 20 months. Imatinib and its metabolites are not excreted via the kidney to a significant extent. In a study of patients with varying degrees of renal dysfunction (mild, moderate and severe - see Table 26 for renal function classification), the mean exposure to imatinib (dose normalized AUC) increased 1.5- to 2-fold compared to patients with normal renal function, which corresponded to an elevated plasma level of AGP, a protein to which imatinib binds strongly. No correlation between imatinib exposure and the severity of renal deficiency was observed. In this study, 800 mg daily was safely used in patients with mild renal dysfunction and 600 mg daily was used in moderate renal dysfunction. The 800 mg dose was not tested in patients with moderate renal dysfunction due to the limited number of patients enrolled. Similarly, only 2 patients with severe renal dysfunction were enrolled at the low (100 mg) dose, and no higher doses were tested. No patients on haemodialysis were enrolled in the study. Literature data showed that a daily dose of 400 mg was well tolerated in a patient with end stage renal disease on haemodialysis. The PK plasma exposure in this patient fell within the range of values of imatinib and its metabolite CGP74588 observed in patients with normal renal function. Dialysis was not found to intervene with the plasma kinetics of imatinib. Since renal excretion represents a minor elimination pathway for imatinib, patients with severe renal insufficiency and on dialysis could receive treatment at the 400 mg starting dose. However, in these patients caution is recommended. The dose can be reduced if not tolerated, or increased for lack of efficacy (see Section 4.4 Special Warnings and Precautions for Use; Section 4.2 Dose and Method of Administration).
Imatinib and its metabolites are not excreted via the kidney to a significant extent. In a study of patients with varying degrees of renal dysfunction (mild, moderate and severe - see Table 26 for renal function classification), the mean exposure to imatinib (dose normalized AUC) increased 1.5- to 2-fold compared to patients with normal renal function, which corresponded to an elevated plasma level of AGP, a protein to which imatinib binds strongly. No correlation between imatinib exposure and the severity of renal deficiency was observed. In this study, 800 mg daily was safely used in patients with mild renal dysfunction and 600 mg daily was used in moderate renal dysfunction. The 800 mg dose was not tested in patients with moderate renal dysfunction due to the limited number of patients enrolled. Similarly, only 2 patients with severe renal dysfunction were enrolled at the low (100 mg) dose, and no higher doses were tested. No patients on haemodialysis were enrolled in the study. Literature data showed that a daily dose of 400 mg was well tolerated in a patient with end stage renal disease on haemodialysis. The PK plasma exposure in this patient fell within the range of values of imatinib and its metabolite CGP74588 observed in patients with normal renal function. Dialysis was not found to intervene with the plasma kinetics of imatinib. Since renal excretion represents a minor elimination pathway for imatinib, patients with severe renal insufficiency and on dialysis could receive treatment at the 400 mg starting dose. However, in these patients caution is recommended. The dose can be reduced if not tolerated, or increased for lack of efficacy (see Section 4.4 Special Warnings and Precautions for Use; Section 4.2 Dose and Method of Administration).
 Chemical name: 4-[(4-methyl-1-piperazinyl) methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl] amino]-phenyl] benzamide methanesulfonate.
Chemical name: 4-[(4-methyl-1-piperazinyl) methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl] amino]-phenyl] benzamide methanesulfonate.