What is in this leaflet
This leaflet answers some common questions about Imrest.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking Imrest against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine.
You may need to read it again.
What Imrest is used for
Imrest is used to help people over 18 years of age with sleeping difficulties, also called insomnia. It can help you fall asleep and to reduce the number of times you wake up during the night. It is used for short term treatment (2-4 weeks) of insomnia. It is not recommended for use for more than 4 weeks at a time.
Your doctor, however, may prescribe Imrest for another purpose.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
Before you take Imrest
When you must not take it
Do not take Imrest if you have:
- been drinking alcohol or you believe that you may have alcohol in your bloodstream
- sleep apnoea (a condition where you temporarily stop breathing while you sleep)
- myasthenia gravis (a condition in which the muscles become weak and tire easily)
- severe liver problems
- acute and/or severe lung problems
- had a stroke
- ever experienced sleep-walking or other unusual behaviour (such as driving, eating, making a phone call or having sex etc.) while not being fully awake after taking Imrest.
Do not take Imrest as a long-term treatment.
Do not take Imrest if you are allergic to it or any of the ingredients listed at the end of this leaflet. Some symptoms of an allergic reaction include skin rash, itching, shortness of breath or swelling of the face, lips or tongue, which may cause difficulty in swallowing or breathing.
Do not give Imrest to children or adolescents. There is no experience with its use in children or adolescents.
Do not take this medicine if you are pregnant. It may affect your developing baby if you take it during pregnancy. Your doctor will discuss the risks and benefits of taking it if you are pregnant.
Do not take it if you are breastfeeding or planning to breastfeed. Imrest passes into breast milk and there is a possibility your baby may be affected. Your doctor will discuss the risks and benefits of using it if you are breastfeeding or planning to breastfeed.
Do not take Imrest after the expiry date (EXP) printed on the pack. If you take it after the expiry date has passed, it may not work as well.
Do not take Imrest if the packaging is damaged or shows signs of tampering.
Before you start to take it
Tell your doctor or pharmacist if you have allergies to:
- any of the ingredients listed at the end of this leaflet
- any other substances such as foods, dyes or preservatives
Tell your doctor if you are pregnant or intend to become pregnant. Like most medicines of this kind, Imrest is not recommended to be used during pregnancy. Your doctor will discuss the risks and benefits of taking it if you are pregnant.
Tell your doctor if you are breastfeeding or planning to breastfeed. Your doctor will discuss the risks of taking it if you are breastfeeding or planning to breastfeed.
Tell your doctor if you have any problems with your breathing or if you often snore while you are asleep.
Tell your doctor if you have ever been addicted to alcohol or any drug or medicine, or if you have ever suffered from a mental illness. If you have, you may be at risk of getting into a regular pattern or habit of taking Imrest.
Tell your doctor if you ever had a history of sleep-walking or other unusual behaviour (such as driving, eating, making a phone call, or having sex etc.) while not being fully awake after taking Imrest. Imrest may cause sleep-walking or other unusual behaviour (such as driving, eating, making a phone call, or having sex etc.) while not being fully awake, some of which have been associated with serious injuries and death. The next morning, you may not remember that you did anything during the night. These activities may occur whether or not you drink alcohol or take other medicines that make you drowsy with Imrest. If you experience any of the above, stop the treatment with Imrest immediately and contact your doctor or health-care provider.
Tell your doctor or pharmacist if you have or have had any medical conditions, especially the following:
- thyroid problems
- depression, psychosis or schizophrenia
- epilepsy
- addiction to drugs or medicines
Tell your doctor if you plan to have surgery.
If you have not told your doctor or pharmacist about any of the above, tell them before you take Imrest.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interfere with the absorption of Imrest. These include:
- medicines to treat depression, anxiety and mental illness
- St John's wort, (hypericum), a herbal remedy for depression
- other medications which may cause drowsiness
- benzodiazepines (medicines used as sedatives or to treat anxiety)
- pain relievers, such as opioids or narcotic analgesics
- alcohol, (ethanol), contained in some medicines e.g. cough syrups
- muscle relaxants
- antihistamines
- medicines used to treat epilepsy
- antiviral medication
- rifampicin, erythromycin or clarithromycin (medicines used to treat infections)
- ketoconazole or itraconazole (medicines used to treat fungal infections)
These medicines may be affected by Imrest or may affect how well it works. You may need to take different amounts of your medicine or different medicines. Your doctor or pharmacist will advise you.
Your doctor or pharmacist has more information on medicines to be careful with or to avoid while taking Imrest.
How to take Imrest
How much to take
Imrest should only be taken when you are able to get a full night's sleep (7 to 8 hours) before you need to be active again.
The standard adult dose of Imrest is one tablet just before you go to bed.
Imrest should be taken in a single intake and not readministered during the same night.
If you are over 65 years of age the dose is half a tablet taken just before you go to bed.
If you have a liver or kidney problem, the usual recommended dose is half a tablet taken just before you go to bed.
Your doctor may have prescribed a different dose.
Ask your doctor if you are unsure of the correct dose for you. They will tell you exactly how much to take.
Follow the instructions they give you. If you take the wrong dose, Imrest may not work as well.
Imrest should not be given to children or adolescents less than 18 years of age.
How to take it
Swallow the tablet with a full glass of water.
When to take it
Take Imrest immediately before you go to bed. This medicine should be taken as a single intake and not be readministered during the same night. It helps put you to sleep quite quickly. If you take Imrest on an empty stomach it may work more quickly.
If you are not sure when to take it, ask your doctor or pharmacist.
How long to take it
Imrest should only be used for short periods (e.g. 2 to 4 weeks).
Continuous long-term use (i.e. longer than 4 weeks) is not recommended.
Ask your doctor or pharmacist if you are not sure how long to take the medicine for.
If you forget to take it
If you forget to take the tablet before you go to bed, and you wake up late in the night or very early in the morning, do not take it.
You may have trouble waking at your normal time.
If you take too much (overdose)
Immediately telephone your doctor or Poisons Information Centre (In Australia call 13 11 26 or New Zealand call 0800 POISON, 0800 764 766) for advice, or go to Accident and Emergency at your nearest hospital, if you think that you or anyone else may have taken too much Imrest.
Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
While you are using Imrest
Things you must do
Tell all the doctors, dentists and pharmacists who are treating you that you are taking Imrest.
If you are about to be started on any new medicine, tell your doctor and pharmacist that you are taking Imrest.
If you plan to have surgery that needs a general anaesthetic, tell your doctor or dentist that you are taking this medicine.
If you become pregnant while you are taking this medicine, stop taking it and tell your doctor or pharmacist immediately.
Things you must not do
Do not take more than the recommended dose unless your doctor tells you to. This can increase the risk of side effects.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not take Imrest to treat any other complaints unless your doctor tells you to.
Do not drink alcohol before or after taking this medicine. This can increase the risk of side effects.
Things to be careful of
Because Imrest will make you sleepy, you should not operate dangerous machinery or drive motor vehicles for 12 hours after you take it. You should also be careful the next morning when you wake up. Make sure you know how you react to Imrest before you drive a car or operate machinery. This is very important if you are taking other drugs that also make you drowsy.
Impairment can occur despite feeling fully awake, in absence of symptoms or if you are feeling better.
Be careful if you are over 65 and unwell or taking other medicines. You may be more sensitive to some of the side effects of Imrest.
You should not drink alcohol while you are taking Imrest. The effects of alcohol could be made worse while taking Imrest.
Side effects
All medicines have some unwanted side effects. Sometimes they are serious, but most of the time they are not. Your doctor or pharmacist has weighed the risks of using this medicine against the benefits they expect it will have for you.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Tell your doctor as soon as possible if you do not feel well while you are taking Imrest.
It helps most people with insomnia, but it may have unwanted side effects in some people.
Tell your doctor if you notice any of the following and they worry you:
- headaches
- dry mouth
- bitter taste in your mouth
- drowsiness
These are the most common side effects of this medicine.
- Less common side effects include:
- heartburn
- nausea, vomiting and/or diarrhoea
- change in appetite
- stomach pain
- rash
- agitation
- depression
- confusion
- anxiety
- dizziness
- blurred vision
- impotence
- sleep walking or other unusual behaviours (such as driving, eating, making a phone call, or having sex etc.) while not being fully awake, some of which have been associated with serious injuries and death.
Alcohol can increase the risk of sleep walking or other behaviours such as driving or eating food whilst asleep. This risk is also increased if you take more than the recommended dose.
Some sleep medicines may cause a short-term memory loss. When this occurs, a person may not remember what has happened for several hours after taking the medicine. This is usually not a problem since most people fall asleep after taking the medicine.
Sleep medicines should in most cases, be used only for short periods of time. If your sleep problems continue, consult your doctor.
Some medicines can cause dependence, especially when they are used regularly for longer than a few weeks. People who have been dependent on alcohol or other drugs in the past may have a higher chance of becoming addicted to sleep medicines. If you have been addicted to alcohol or drugs in the past, it is important to tell your doctor before starting Imrest.
If any of the following happen, stop taking this medicine and tell your doctor immediately, or go to Accident and Emergency at your nearest hospital:
- swelling of the face, lips, mouth or throat, which may cause difficultly in swallowing or breathing
- hives
- fainting
These are very serious side effects. If you have them, you may have had a serious allergic reaction to Imrest. You may need urgent medical attention or hospitalisation.
These side effects are very rare.
Tell your doctor immediately, or go to Accident and Emergency at your nearest hospital if you feel you are becoming depressed, having suicidal thoughts or are experiencing changes in your behaviour.
Tell your doctor or pharmacist if you notice anything else that is making you feel unwell. Other side effects not listed above may occur in some consumers.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
After using Imrest
Sometimes when medicines are stopped suddenly, after being used for a long time, withdrawal symptoms may occur. Symptoms of withdrawal may include abdominal and muscle cramps, vomiting and sweating.
In some cases your insomnia may appear worse for a short time; speak to your doctor if this occurs.
Tell your doctor if you have any problems when you stop taking Imrest.
If you have any queries about any aspect of your medicine, or any questions regarding the information in this leaflet, discuss them with your doctor or pharmacist.
Storage
Keep your tablets in the blister pack until it is time to take them. If you take the tablets out of the box or the blister pack they may not keep well.
Keep your tablets in a cool dry place where the temperature stays below 25°C.
Do not store Imrest or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
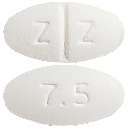
Imrest is a white, film-coated, oval tablet embossed "Z" breakline "Z" on one side and "7.5" on the reverse.
Each pack contains 30 tablets.
Ingredients
The active ingredient in Imrest is zopiclone. Each tablet contains 7.5 mg of zopiclone.
The tablets also contain the following inactive ingredients:
- lactose
- calcium hydrogen phosphate
- maize starch
- povidone
- magnesium stearate
- Opadry White Y-1-7000.
Supplier
Imrest is supplied in Australia by:
Alphapharm Pty Ltd trading as Viatris
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.viatris.com.au
Phone: 1800 274 276
This leaflet was prepared in
December 2021.
Australian registration numbers:
AUST R 99794
Imrest® is a Viatris company trade mark
Imrest_cmi\Dec21/00
Published by MIMS January 2022

 The chemical name for zopiclone is 6-(5-chloro-2-pyridyl)-6,7-dihydro-7-oxo-5H-pyrrolo[3,4-b]pyrazin-5-yl-4-methylpiperazine-1-carboxylate.
The chemical name for zopiclone is 6-(5-chloro-2-pyridyl)-6,7-dihydro-7-oxo-5H-pyrrolo[3,4-b]pyrazin-5-yl-4-methylpiperazine-1-carboxylate.