What is in this leaflet
This leaflet answers some common questions about Isoptin.
It does not contain all the available information.
It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking Isoptin against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, talk to your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What Isoptin is used for
There are two types of Isoptin:
- Isoptin tablets (available as 40 mg and 80 mg tablets)
- Isoptin SR (available as 180 mg and 240 mg tablets)
The letters SR in the name Isoptin SR stand for "sustained release". This means the medicine is released into the blood over an extended period of time, usually allowing the medicine to be taken once a day.
Depending on your condition, your doctor will prescribe Isoptin or Isoptin SR.
In most parts of this leaflet, the name Isoptin is used to refer to both Isoptin tablets and Isoptin SR tablets. Where there is information specific to the type of Isoptin, the separate names are used.
Isoptin and Isoptin SR are used in the treatment of:
- high blood pressure, also called hypertension
- angina (chest pain)
Isoptin tablets are also used to treat irregular heartbeats, also called arrhythmias
Your doctor may have prescribed Isoptin for another reason. Ask your doctor if you have any questions about why Isoptin has been prescribed for you.
Isoptin and Isoptin SR belong to a group of medicines called calcium channel blockers or calcium antagonists. They work by opening up blood vessels, which lets more blood and oxygen reach the heart and at the same time lowers high blood pressure. Isoptin tablets also help to control fast or irregular heartbeats.
Isoptin does not change the amount of calcium in your blood or bones. Calcium in your diet or in calcium supplements will not interfere with the way Isoptin works.
Isoptin SR is not recommended for use in children under the age of 18, as there have been no studies of its effects in this age group.
Isoptin is available only with a doctor's prescription.
There is no evidence that Isoptin is addictive.
Before you take Isoptin
When you must not take it
Do not take Isoptin if you have an allergy to verapamil hydrochloride or any of the ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin
Do not take Isoptin if you:
- have certain heart conditions (such as heart failure, a very slow heart rate, heart conduction problems, some irregular heartbeats or disease of the heart muscle)
- have low blood pressure, also called hypotension
- are taking any of the following medications, or medications containing these ingredients:
- ivabradine
- Direct Oral Anticoagulants (DOACs) such as dabigatran (in certain situations)
Do not use Isoptin after the expiry date (EXP) printed on the pack. If you take this medicine after the expiry date has passed, it may not work as well.
Do not use Isoptin if the packaging is torn or shows signs of tampering.
If you are not sure whether you should start taking Isoptin contact your doctor.
Before you start to take Isoptin:
Tell your doctor if you have allergies to:
- any other medicines
- any other substances, such as foods, dyes or preservatives.
Tell your doctor if you have or have ever had any medical conditions especially the following:
- any other heart problem
- blood vessel (circulatory) disease or a stroke
- liver or kidney problems
- muscle conditions such as Duchenne muscular dystrophy, myasthenia gravis, Lambert-Eaton syndrome
Tell your doctor if you are pregnant or plan to become pregnant. Isoptin may affect your baby if you take it in pregnancy. Your doctor will discuss the possible risks and benefits of taking Isoptin during pregnancy.
Tell your doctor if you are breastfeeding or plan to breastfeed. Isoptin passes into breast milk. Your doctor will discuss the possible risks and benefits of taking Isoptin when breastfeeding.
If you have not told your doctor about any of the above, tell them before you start taking Isoptin.
Taking other medicines
Several medicines can cause unwanted reactions if used with Isoptin.
Ask your doctor or pharmacist for advice before taking this medicine.
In particular tell your doctor if you are taking any of the following medicines:
Medicines to treat heart problems or high blood pressure:
- Beta-blockers e.g. atenolol, propranolol, metoprolol, etc
- Ivabradine
- Digoxin
- Any other medicines used to control an irregular heartbeat e.g. quinidine, flecainide, amiodarone, disopyramide
- Any medicines used to control high blood pressure (especially prazosin or terazosin)
Medicines used to lower cholesterol:
- Statins such as atorvastatin or simvastatin
Medicines used to treat or prevent blood clots (sometimes referred to as "blood thinners")
- Direct Oral Anticoagulants (DOACs) such as dabigatran
- Aspirin
Medicines used to treat or prevent gout:
- Colchicine, sulfinpyrazone.
Medicines used to treat psychological problems
- Any medicines to treat depression, or psychosis. Such as imipramine, buspirone, midazolam or lithium
Medicines to treat epilepsy or seizures:
- Phenytoin, carbamazepine, phenobarbital.
Medicines to treat or prevent organ transplant rejection:
- Cyclosporin, everolimus, sirolimus, tacrolimus
Medicines used to treat infections or tuberculosis:
- such as erythromycin, clarithromycin, telithromycin or rifampicin
Medicines used in the treatment of Human Immunodeficiency Virus (HIV):
- such as ritonavir
Medicines used in surgical procedures:
- General anaesthetics used for inducing sleep
- Muscle relaxants
Other medicines that may react with Isoptin:
- theophylline, a medicine used to treat asthma
- doxorubicin, a medicine used to treat certain cancers
- cimetidine, a medicine commonly used to treat stomach ulcers and reflux
- metformin and glibenclamide, medicines used to treat diabetes
- aspirin
Avoid alcohol while using Isoptin. You may experience greater blood pressure lowering effects than usual.
Avoid grapefruit juice, as this may increase the blood levels of verapamil.
These medicines may be affected by Isoptin, or may affect how well it works. You may need different amounts of your medicine or you may need to take different medicines. Your doctor will advise you.
This is not a complete list of medicines which may interfere with Isoptin.
Tell your doctor or pharmacist if you are taking or have recently taken any other medicines or herbal remedies, including those obtained without a prescription from a pharmacy, supermarket or health food shop.
How to take Isoptin
How much to take
Your doctor will tell you how many tablets you will need to take each day and when to take them. This depends on your condition and whether or not you are taking any other medicines.
Isoptin tablets are usually taken two or three times a day.
The usual dose of Isoptin SR is once daily or they may be taken twice daily.
Follow all directions given to you by your doctor carefully.
If you do not understand the instructions on the pharmacist's label fixed on the box, ask your doctor or pharmacist for help.
How to take it
Swallow Isoptin with a glass of water.
Isoptin 40 mg and 80mg tablets are to be swallowed whole. They are not meant to be broken.
Isoptin SR tablets can be broken in half if your doctor has prescribed half a tablet.
Do not crush or chew Isoptin SR tablets.
When to take it
Take Isoptin SR with food.
Isoptin 40 mg and 80 mg tablets can be taken with or without food.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to. Otherwise, take it as soon as you remember, and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for the dose that you missed. This may increase the chance of you getting an unwanted side effect.
If you miss more than one dose, or are not sure what to do, check with your doctor or pharmacist.
How long to take it
Treatment with Isoptin is usually long term. Keep taking Isoptin for as long as your doctor recommends.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at your nearest hospital, if you think that you or anyone else may have taken too much Isoptin. Do this even if there are no signs of discomfort or poisoning.
You may need urgent medical attention.
If you take too much Isoptin, you may have difficulty breathing, a slow heartbeat, chest pain, feel very faint or collapse; however, if you are taking Isoptin SR (sustained release) tablets the medicine is released into the blood over an extended period of time, so you may not notice these symptoms immediately.
While you are taking Isoptin
Things you must do
If you become pregnant while taking Isoptin, tell your doctor.
Tell all doctors, dentists and pharmacists who are treating you that you are taking Isoptin.
If you are being treated for angina, tell your doctor if you continue to have angina attacks or if they become more frequent while you are using Isoptin.
If you are going to have surgery including dental surgery, tell your doctor or dentist that you are taking Isoptin.
Visit your doctor regularly so that they can check on your progress.
Your doctor may ask you to have blood tests to check your liver from time to time.
Things you must not do
Do not take Isoptin with grapefruit or its juice.
Do not give Isoptin to anyone else, even if they have the same condition as you.
Do not take Isoptin to treat any other complaints unless your doctor tells you to.
Do not stop taking Isoptin, or lower the dosage without checking with your doctor.
Things to be careful of
Be careful getting up from a sitting position. Dizziness, light-headedness or fainting may occur, especially when you get up quickly. Getting up slowly may help.
Be careful driving or operating machinery until you know how Isoptin affects you. As with other medicines, Isoptin may cause dizziness, light-headedness or tiredness in some people. If this occurs, do not drive, operate machinery or do anything else that could be dangerous if you are tired, dizzy or lightheaded.
If you drink alcohol while taking Isoptin, dizziness or light-headedness may be worse.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Isoptin.
Like all other medicines, Isoptin may have unwanted side effects in some people. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
As with most medicines, if you are over 65 years of age you may have an increased chance of getting side effects. Report any side effects to your doctor promptly.
Ask your doctor or pharmacist any questions you may have.
Tell your doctor if you notice any of the following more common side effects and they worry you:
- constipation
- dizziness, light-headedness
- feeling sick, upset stomach
- headache
- tiredness
- flushing
Tell your doctor immediately or go to Accident and Emergency at the nearest hospital if you notice any of the following:
- chest pain, fainting, collapse
- slow, fast, or irregular heart beat
- shortness of breath (sometimes with tiredness, weakness and reduced ability to exercise), which may occur together with swelling of the feet and legs due to fluid build up
- fever, upper stomach pain, feeling generally unwell
- severe blisters, skin rash, itching or flaking skin
Other side effects not listed above may occur in some patients. Ask your doctor or pharmacist for more information about side effects, as they have a more complete list of side effects. Inform your doctor promptly about these or any other symptoms. If the condition persists or worsens, seek medical attention.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Tell your doctor if you notice anything that is making you feel unwell.
After using Isoptin
Storage
Keep your tablets in the pack until it is time to take them. If you take the tablets out of the pack they will not keep well.
Keep Isoptin tablets in a cool dry place where the temperature stays below 25°C.
Do not store Isoptin or any other medicine in the bathroom or near a sink.
Do not leave it in the car on hot days or on window sills. Heat and dampness can destroy some medicines.
Keep your tablets where children cannot reach them.
Disposal
Medicines should not be disposed of via wastewater or household waste.
If your doctor tells you to stop taking Isoptin, or the tablets have passed their expiry date, ask your pharmacist what to do with any tablets left over.
Product description
What it looks like
Isoptin is available as:
Isoptin 40 mg: White, biconvex film coated tablets, embossed "40" on one side and the Knoll triangle on the other side.
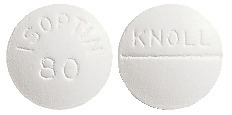
Isoptin 80 mg: White, biconvex film coated tablet embossed "Isoptin 80" on the front and "Knoll" above the score on the back.
Each pack of Isoptin 40 mg and Isoptin 80 mg contains 100 tablets.
Isoptin SR is available as:
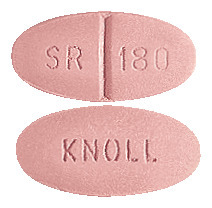
Isoptin 180 SR: Old rose, oval film coated tablet. KNOLL on one face and 'SR' score '180' on the other face.
Isoptin 240 SR: Light green, oblong film-coated tablet (curved tooling). Score and 2 logos on one face and score on the other face.
Each pack of Isoptin 180 SR and Isoptin 240 SR contains 30 tablets.
Ingredients
Isoptin tablets and Isoptin SR tablets are gluten free.
The active ingredient in Isoptin is verapamil hydrochloride.
Isoptin tablets contain 40 mg or 80 mg of verapamil hydrochloride.
These tablets also contain:
- calcium hydrogen phosphate dihydrate
- microcrystalline cellulose
- croscarmellose sodium
- magnesium stearate
- colloidal anhydrous silica
- hypromellose
- purified talc
- sodium lauryl sulfate
- macrogol 6000
- titanium dioxide
Isoptin 180 SR tablets contain 180mg of verapamil hydrochloride.
These tablets also contain:
- sodium alginate
- microcrystalline cellulose
- povidone
- magnesium stearate
- hypromellose
- macrogol 400
- macrogol 6000
- purified talc
- titanium dioxide
- iron oxide red
- glycol/butylene glycol montanate
Isoptin 240 SR tablets contain 240mg verapamil hydrochloride.
Isoptin 240 SR tablets also contain:
- sodium alginate
- powdered cellulose
- povidone
- magnesium stearate
- hypromellose
- purified talc
- macrogol 400
- macrogol 6000
- titanium dioxide
- quinoline yellow
- indigo carmine
- glycol/butylene glycol montanate
- purified water
Supplier
Isoptin tablets are supplied by:
Mylan Health Pty Ltd
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point, NSW 2000
Australia
www.mylan.com.au
Phone: 1800 314 527
Australian registration numbers:
Isoptin 40 mg - AUST R 65502
Isoptin 80 mg - AUST R 65503
Isoptin 180 SR - AUST R 54032
Isoptin 240 SR - AUST R 12801
This leaflet was prepared in July 2021.
Isoptin_cmi\Jul21/00
Published by MIMS August 2021