What is in this leaflet
This leaflet answers some common questions about ITRANOX capsules. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking ITRANOX capsules against the benefits this medicine is expected to have for you. If you have any concerns about taking ITRANOX capsules, ask your doctor or pharmacist.
Keep this leaflet with your medicine. You may need to read it again.
What ITRANOX capsules are used for
ITRANOX capsules are used to treat certain fungal infections which include the following:
- persistent infections of the nails, skin, hands, feet or groin;
- persistent candida (yeast) infections of the vagina;
- eye infections which have not responded to other treatment or which may be affecting vision;
- candida (yeast) infections of the mouth or throat in patients with lower resistance to disease;
- generalised infections.
ITRANOX works by killing or stopping the growth of the fungus that causes the infection.
Your doctor may have prescribed ITRANOX capsules for another reason.
Ask your doctor if you have any questions about why this medicine has been prescribed for you.
Before you take ITRANOX capsules
When you must not take it
Do not take ITRANOX capsules if:
- you are pregnant or may become pregnant.
If there is any chance of you becoming pregnant, talk to your doctor about the need for highly effective contraception. Once you have finished taking ITRANOX, you should continue using highly effective contraception until you have had your next period. Tell your doctor immediately if you do become pregnant while taking ITRANOX. - you have a condition called heart failure (also called congestive heart failure or CHF)
ITRANOX could make it worse. If your doctor decides that you need to take ITRANOX even if you have this condition, be sure to get immediate medical help if you have shortness of breath, unexpected weight gain, swelling of the legs, unusual fatigue, or begin to wake up at night. - you have an allergy to ITRANOX capsules or any of the ingredients.
See Product Description at the end of this leaflet.
ITRANOX capsules must not be taken with certain medicines. Do not take ITRANOX capsules with any of the following:
- terfenadine, astemizole or mizolastine (used for allergy or hay fever);
- bepridil, felodipine, lercanidipine, ivabradine, ranolazine, eplerenone and nisoldipine (used to treat angina (crushing check pain) or high blood pressure);
- domperidone (used to treat nausea and vomiting);
- ticagrelor (used for the prevention of heart attack or stroke);
- cisapride (used for certain digestive problems);
- certain medicines used to produce calmness or to help you sleep (midazolam (oral) or triazolam);
- simvastatin, lomitapide or lovastatin (used to lower your cholesterol);
- lurasidone, pimozide or sertindole (used to treat mental disorders);
- disopyramide, dronedarone, quinidine or dofetilide (used to treat irregular heartbeats);
- levacetylmethadol, methadone (used for severe pain or to manage opioid-dependency);
- tricagrelor (used for the prevention of heart attack or stroke);
- dihydroergotamine and ergotamine (used to treat migraine);
- ergometrine or methylergometrine (used to control bleeding and maintain uterine contraction after child birth);
- halofantrine (used to treat malaria);
- irinotecan, an anti-cancer medicine;
- isavuconazole (used to treat fungal infections);
- naloxegol (used to treat constipation caused by taking opioid painkillers);
- avanafil (used to treat erectile dysfunction);
- dapoxetine (used to treat premature ejaculation);
- eliglustat (if you know you do not break down drugs that are broken down by the enzyme known as CYP2D6, you should check with your doctor if you can take this medicine).
- finerenone (used to treat kidney problems in patients with type 2 diabetes);
- voclosporin (used to treat lupus-related kidney problems).
Medicines not recommended while you are on ITRANOX capsules, when you are on a stable dose of this medicine: venetoclax
Wait at least 2 weeks after stopping ITRANOX capsules before starting this medicine unless your doctor feels it is necessary. Taking ITRANOX capsules.
If you have kidney or liver problems, do not take ITRANOX capsules with any of the following:
- colchicine (used to treat gout);
- fesoterodine or solifenacin (used to control irritated urinary bladder);
- telithromycin (an antibiotic).
Do not take ITRANOX capsules if the packaging is torn or shows signs of tampering.
Do not take ITRANOX capsules beyond the expiry date (month and year) printed on the pack.
Before you start to take it
You must tell your doctor if:
- you are breast feeding or wish to breastfeed;
- you have had an allergic reaction to other medicines used to treat fungal infections;
- you have or have had any liver problems;
- you have or have had any kidney problems;
- you have heart problems
- you are a neutropenic, AIDS or an organ transplant patient.
- you are a cystic fibrosis patient. If you have not told your doctor or pharmacist about any of the above, tell them before you start taking or are given ITRANOX capsules.
Your doctor will advise whether or not to take ITRANOX or if you need to adjust the dose or adapt your treatment.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including medicines you can buy without a prescription from a pharmacy, supermarket or health food shop.
In particular, ITRANOX capsules must not be taken with some medicines. Examples are:
- terfenadine, astemizole or mizolastine (used for allergy or hay fever);
- bepridil, felodipine, nisoldipine, lercanidipine, ivabradine, ranolazine, eplerenone (used to treat angina (crushing chest pain) or high blood pressure);
- cisapride (used for certain digestive problems);
- certain medicines used to produce calmness or to help you sleep (midazolam (oral) or triazolam);
- simvastatin, lomitapide or lovastatin (used to lower your cholesterol);
- lurasidone, pimozide or sertindole (used to treat mental disorders);
- disopyramide, dronedarone, quinidine or dofetilide (used to treat irregular heartbeats);
- levacetylmethadol, methadone (used for severe pain and to manage opioid-dependency);
- ticagrelor (used for the prevention of heart attack or stroke);
- dihydroergotamine and ergotamine (used to treat migraine);
- ergometrine or methylergometrine (used to control bleeding and maintain uterine contraction after child birth);
- artemether-lumefantrine, halofantrine, quinine (used to treat malaria);
- irinotecan, an anti-cancer medicine;
- domperidone (used to treat nausea and vomiting);
- isavuconazole (used to treat fungal infections);
- naloxegol (used to treat constipation caused by taking opioid painkillers);
- avanafil (used to treat erectile dysfunction);
- dapoxetine (used to treat premature ejaculation);
- eliglustat (if you know you do not break down drugs that are broken down by the enzyme known as CYP2D6, you should check with your doctor if you can take this medicine).
Medicines that must never be taken while you are taking ITRANOX capsules, if you have kidney or liver problems:
- colchicine (used to treat gout);
- fesoterodine or solifenacin, when used to control irritated urinary bladder;
- telithromycin (an antibiotic).
If you have chronic lymphocytic leukemia/small lymphocytic lymphoma and you want to newly start this medicine or are making dose adjustments:
- venetoclax (used to treat certain cancers).
Wait at least 2 weeks after stopping ITRANOX capsules before taking any of these medicines.
Certain medicines are not recommended because they may be affected by ITRANOX capsules or may affect how well ITRANOX capsules work. Your doctor may need to adjust the dose or adapt your treatment. Examples of these medicines are:
- phenytoin, phenobarbital or carbamazepine (used to treat fits);
- bedaquiline, delamanid, rifampicin, rifabutin or isoniazid (used to treat tuberculosis);
- certain medicines used to treat HIV/AIDS, such as cobicistat, boosted elvitegravir, efavirenz, indinavir, maraviroc, nevirapine, saquinavir and ritonavir, boosted darunavir, ritonavir-boosted fosamprenavir, tenofovir disoproxil fumerate (TDF);
- boosted asunaprevir, boceprevir, daclatasvir, telaprevir, vaniprevir (used to treat hepatitis C);
- certain antineoplastics such as axitinib, bosutinib, bortezomib, brentuximab vedotin, busulphan, carbazitaxel, cabozanitinib, ceritinib, cobimetinib, crizotinib, dabrafenib, dasatinib, docetaxel, erlotinib, gefitinib, glasdegib, ibrutinib, idelalisib, imatinib, ixabepilone, lapatinib, nilotinib, nintedanib, olaparib, panobinstat, pazopanib, ponatinib, regorafenib, ruxolitinib, sunitinib, sonidegib, talazoparib, trabectedin, trastuzumab emtansine, vandetanib, vinca alkaloids (used to treat certain cancers);
- sunitinib (used to treat certain types of stomach, bowel, or oesophagus tumour and kidney or pancreatic cancer);
- gefitinib (used to treat breast, lung and other cancers);
- imatinib (used to treat lung cancer);
- aliskiren, diltiazem (to treat hypertension);
- bosentan, digoxin, nadolol, riociguat, and certain calcium channel blockers including amlodipine besylate, nifedipine, nimodipine, other dihydropyridines and verapamil (used to treat heart or blood pressure problems);
- vorapaxar (used to treat heart attacks or strokes);
- atorvastatin (used to lower cholesterol);
- anticoagulants such as apixaban, coumarins & coumarin-like medicines (e.g. warfarin), cilostazol, dabigatran, rivaroxaban (used to slow blood clotting);
- alfuzosin, dutasteride, soldosin (used to treat Benign Prostatic enlargement);
- sildenafil (used to treat erectile dysfunction or pulmonary hypertension);
- tadalafil, udenafil, vardenafil (used to treat erectile dysfunction);
- colchicine (used to treat gout);
- conivaptan, tolvaptan (used to treat low blood sodium levels);
- mozavaptan; to treat low blood sodium;
- fentanyl, a strong medicine for pain;
- alfentanil, buprenorphine, oxycodone, sufentanil (used in surgery for pain relief and to help anaesthesia);
- meloxicam, to treat joint inflammation and pain;
- salmeterol (to improve breathing)
- darifenacin, fesoterodine, imidafenacin, oxybutynin, tolterodine (used to treat urinary incontinence);
- tamsulosin (used to treat male urinary incontinence)
- ciprofloxacin, clarithromycin, erythromycin, telithromycin (antibiotics);
- methylprednisolone, budesonide, ciclesonide, fluticasone and dexamethasone (often used for conditions such as inflammations, asthma and allergies);
- bilastine, ebastine, rupatadine (used to treat allergies);
- everolimus (given after an organ transplant)
- cyclosporin, rapamycin (also known as sirolimus), tacrolimus, temsirolimus (used to help prevent organ transplant rejection or to treat certain problems with the immune system);
- trimetrexate (used to treat certain type of pneumonia);
- buspirone, perospirone, ramelteon, midazolam IV, alprazolam, brotizolam (used to treat anxiety or help you sleep);
- aripiprazole, cariprazine, haloperidol, quetiapine, risperidone; to treat psychosis;
- medicines taken for diabetes (in particular repaglinide and saxagliptin);
- aprepitant, netupitant (used for nausea and vomiting during cancer treatment)
- praziquantel, (used to treat fluke and tapeworms);
- some contraceptive pills (birth control pills), such as dienogest, ulipristal;
- reboxetine, venlafaxine (used to treat depression and anxiety);
- cinacalcet, to treat an over active parathyroid;
- alitretinoin (oral formulation), to treat eczema;
- eletriptan (used to treat migraine);
- medicines which neutralize stomach acid or suppress the production of stomach acid (such as antacids, cimetidine, ranitidine, omeprazole);
- Saccharomyces boulardii, loperamide (used to treat diarrhea);
- lumacaftor/ ivacaftor (used to treat Cystic Fibrosis);
- guanfacine (used to treat Attention Deficit Hyperactivity Disorder);
- suvorexant, zopiclone (used to treat insomnia);
- galantamine (used to treat Alzheimer’s disease)
- glecaprevir/pibrentasvir; elbasvir/grazoprevir; ombitasvir/paritaprevir/ritonavir (with or without dasbuvir) combinations to treat Hepatitis C
- cabergoline (used to treat Parkinson's Disease;
- cannabinoids (used to treat nausea and vomiting, weight loss for patients with immune system problems and muscle spasms in patients with Multiple Sclerosis)
- artemether-lumefantrine, quinine (used to treat malaria);
Taking ITRANOX capsules
How much to take
Adults
The usual doses are shown below, but your doctor may decide to adjust them for your individual needs.
Tinea of body & groin:
1 capsule (100 mg) daily for 2 weeks.
Tinea of hands & feet:
1 capsule (100 mg) daily for 4 weeks.
Other skin infections:
2 capsules (200 mg) daily for 1 week.
Eye infections:
2 capsules (200 mg) daily for 3 weeks.
Vaginal infections:
2 capsules (200 mg) morning & evening for 1 day, or 2 capsules (200 mg) daily for 3 days.
Mouth infections:
1 capsule (100 mg) daily for 4 weeks or 2 capsules (200 mg) daily for 4 weeks.
Systemic infections:
1 to 2 capsules (100 mg to 200 mg) once or twice daily for 3 weeks to 8 months, depending on the condition.
Nail infections:
Continuous nail therapy
2 capsules (200 mg) once daily for 3 months.
Cyclic (pulse) nail therapy
2 capsules twice daily for 1 week.
After that, stop taking ITRANOX for 3 weeks. Then the cycle is repeated, once for fingernails and twice for toenail infections (with or without fingernail infections). (See below).
Fingernails only
Week 1: Take 2 capsules twice daily.
Week 2, 3, 4: No ITRANOX.
Week 5: Take 2 capsules twice daily.
Week 6: Stop.
Toenails with or without fingernails
Week 1: Take 2 capsules twice daily.
Week 2, 3, 4: No ITRANOX.
Week 5: Take 2 capsules twice daily.
Week 6, 7, 8: No ITRANOX.
Week 9: Take 2 capsules twice daily.
Week 10: Stop.
Children and Elderly
ITRANOX capsules are not recommended for use in children and in the elderly.
How to take it
Swallow the capsules whole.
Always take ITRANOX capsules after a meal.
Do not take medicines that neutralise stomach acid within 2 hours of taking ITRANOX capsules. This is because sufficient stomach acid is required to ensure that ITRANOX capsule is properly absorbed by the body. If you take medicines that suppress the production of stomach acid, you should take your ITRANOX capsules with an acidic drink, such as a cola beverage.
If you forget to take it
Take the dose you missed as soon as you remember, and then continue to take it as you would normally.
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Do not take a double dose to make up for the one you missed. If you have missed more than one dose, or are not sure what to do, check with your doctor or pharmacist.
If you have trouble remembering when to take your medicine, ask your pharmacist for some hints.
If you have taken too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre for advice or go to Accident and Emergency at your nearest hospital. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
Poisons Information Centre telephone numbers: 13 11 26 (Australia).
While you are taking ITRANOX capsules
Things you must do
Always follow your doctor's instructions carefully.
If you have to take ITRANOX capsules continuously for more than 1 month, your doctor may ask you to have your blood checked regularly. This is to make sure that your liver is not affected. Signs and symptoms may include unusual fatigue, anorexia, nausea and/or vomiting, jaundice, dark urine or pale stool.
Tell your doctor immediately if you do become pregnant while taking ITRANOX. If there is any chance of you becoming pregnant, talk to your doctor about the need for highly effective contraception. Once you have finished taking ITRANOX, you should continue using highly effective contraception until you have had your next period.
If you are about to start taking a new medicine, tell your doctor and pharmacist that you are taking ITRANOX capsules.
Always complete the treatment as directed by your doctor, even if the signs of infection have gone.
Things you must not do
Do not take ITRANOX capsules to treat any other complaint unless your doctor says so.
Do not give this medicine to anyone else, even if his or her symptoms seem similar to yours.
Things to be careful of
Be careful driving or operating machinery. You may feel dizzy while taking ITRANOX capsules. If you experience this or similar effects, you should avoid driving and using machines.
Make sure you know how you react to ITRANOX capsules before you drive a car, operate machinery or do anything else that could be dangerous if you are dizzy or lightheaded.
Side Effects
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some side effects. Do not be alarmed by this list of possible side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you experience any of the following:
- upset stomach, stomach pain or discomfort, bloating, nausea, vomiting, diarrhoea, constipation, an unpleasant taste in your mouth.
- shortness of breath, headache, dizziness, fever.
- confusion
- cough, chills, cold or flu-like symptoms
- a change in menstrual pattern.
- Inflammation of sinus or nose
- unusual hair loss or thinning.
- erectile dysfunction.
- muscle weakness or pain, painful joints, tremors.
- High or low blood pressure
- Sleepiness
- Excessive sweating
- Inflammation of the pancreas.
Tell your doctor immediately if you notice any of the following as you may need urgent medical care:
- tingling, numbness or weakness in the hands or feet.
- increased heart rate
- swelling of hands ankles, feet, legs or abdomen.
- shortness of breath, unexpected weight gain, unusual fatigue, or begin to wake up at night.
- oversensitivity to sunlight.
- blurry or double vision, ringing in the ears.
- lose the ability to control your bladder or urinate much more than usual.
STOP taking ITRANOX capsules and tell your doctor immediately or go to Accident and Emergency at your nearest hospital if any of the following happen:
- abnormal tiredness, loss of appetite, nausea, vomiting, abdominal pain, dark urine, pale stools, yellowing of the skin or eyes (liver disorder).
- sudden signs of allergy such as rash, itching or hives on the skin, swelling of the face, lips, tongue or other parts of the body, shortness of breath or difficulty breathing, wheezing or trouble breathing.
- a severe skin disorder (widespread rashes with peeling skin and blisters in the mouth, eyes and genitals, or rashes with small pustules or blisters).
- you experience any hearing loss symptoms. In very rare cases, patients taking ITRANOX have reported temporary or permanent hearing loss.
Other side effects not listed above may also occur in some people. Tell your doctor if you notice any other effects.
After using ITRANOX capsules
Storage
Keep ITRANOX capsules in the pack until it is time to take them.
Keep ITRANOX capsules in a cool dry place where the temperature is below 25°C.
Keep your medicines where young children cannot reach them. A locked cupboard at least one-and-a-half metres (1.5 m) above the ground is good place to store medicines.
Do not store ITRANOX capsules, or any other medicine, in the bathroom or near a sink. Do not leave medicines in the car or on windowsills. Heat and dampness can destroy some medicines.
Disposal
If your doctor tells you to stop taking ITRANOX capsules or your medicines have passed its expiry date, ask your pharmacist what to do with any medicine which may be left over.
Product Description
What it looks like
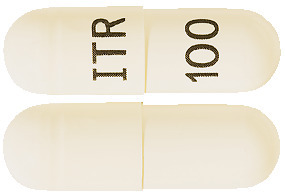
ITRANOX capsules are size “0el” hard gelatin white opaque capsules filled with off-white to cream coloured pellets and imprinted with ‘ITR’ on cap and ‘100’ on body. AUST R 244432.
They are supplied in a PVC/PE/PVDC/Aluminium blister packs of 15, 28 or 60 capsules.
Ingredients
The active ingredient in each ITRACONAZOLE GPPL capsule is 100 milligrams of itraconazole.
Other ingredients include:
- hypromellose
- macrogol 20,000
- sucrose
- maize starch
- hard gelatin capsules printed with TekPrint SW-9008 black ink.
This medicine contains sugars and sulfites.
Distributor
Arrotex Pharmaceuticals Pty Ltd
15 – 17 Chapel Street
Cremorne, VIC 3121
www.arrotex.com.au
This leaflet was prepared in September 2023.
Published by MIMS November 2023