What is in this leaflet
This leaflet answers some common questions about KLYX Enema. It does not contain all the available information.
It does not take the place of talking to your doctor or pharmacist.
If you have any concerns about taking this medicine, ask your doctor, nurse or pharmacist.
Keep this leaflet with the medicine.
You may need to read it again.
What KLYX Enema is used for
KLYX Enema is used when oral therapies are not suitable, for:
- Treatment of faecal impaction or constipation
- Colon evacuation prior medical procedures.
It acts by increasing water content in the stools, thereby softening hard stools, facilitating opening of the bowels and cleansing the bowel area. It starts to work approximately 5-20 minutes after application.
If you have constipation it is important to also drink plenty of water and to increase fibre in diet, except if the constipation is caused by certain medications such as codeine.
Before you use KLYX Enema
Do not use KLYX Enema if:
- you are hypersensitive (allergic) to the active substances or any other ingredients found in KLYX Enema
- you are suffering from active inflammatory bowel disease
- you have intestinal bleeding (including haemorrhoids or anal fissures)
- you are suffering from abdominal pain of unknown origin
- you have a bowel obstruction.
Take special care with KLYX Enema
KLYX Enema should only be used on a short-term basis. Do not use for more than one week. KLYX Enema should not be used regularly. Regular use may cause irritation in the bowel, lead to dehydration and cause chemical imbalances in your blood.
Prolonged use of laxatives is undesirable and may lead to dependence.
Seek medical advice if your constipation persists.
Tell your doctor if you are taking other medications or have a particular diet or allergies to any, foods, preservatives or dyes.
Pregnancy
Ask your doctor or pharmacist for advice before taking any medicine. No untoward effects on the fetus have been seen.
Breast-feeding
Ask your doctor or pharmacist for advice before taking any medicine. No untoward effects of KLYX Enema have been seen in the suckling infant.
Driving and using machinery
KLYX Enema is not expected to affect your ability to drive a car or operate machinery.
After using KLYX Enema, ensure you have an available toilet nearby.
How to use KLYX Enema
KLYX enema is used rectally as directed by your health professional. If requested by your healthcare professional KLYX enema can be repeated once a day for up to maximum of 2 days for bowel cleansing and 6 days for difficult to treat constipation.
How much to use
Adults and children 12 years and over: Normally 120 mL is effective.
Children from 4 to 6 years:
Normally 60 mL (half of the contents of KLYX enema 120 mL) is effective.
Children from 7 to 11 years:
60 mL (half of the contents of KLYX Enema 120 mL) may be effective. Up to one KLYX 120 mL may be administered (see How to administer KLYX Enema below).
The use of more than one enema should be strictly under the supervision of your healthcare professional.
If you believe that the effects of KLYX Enema on your bowel movement is too strong, or the product has not had the desired effect, speak with your doctor, nurse or pharmacist.
If you do not understand the instructions in this leaflet, ask your doctor, nurse or pharmacist for help.
How to administer KLYX Enema
Do not attempt to use KLYX Enema until you have checked the following illustrations, and have read and understood the accompanying instructions.
KLYX Enema may be warmed up to body temperature in a water-bath before it is used.
Left-side position:
Lie on left side with knee bent, arms resting comfortable.
Knee-chest position:
Kneel, then lower head and chest forward until left side of face resting on surface with left arm folded comfortable.
Follow these simple steps:
CAUTION: REMOVE THE PROTECTIVE CAP FROM THE APPLICATOR BEFORE INSERTING.
- Hold bottle upright. Closely examine the applicator and bottle. For protection, the applicator is provided with a cap.

- Grasp the applicator cap with other hand, pull gently to remove it.

- Twist to remove the upper-most end of the applicator.

- The applicator is now ready to use.

- Lubricate the tip of the enema with small amounts of Vaseline or KY jelly, or by pressing out some drops of KLYX Enema, and letting them run down the tip.
- While laying on your side or in the 'knee-chest position', gently insert the tip of the enema into the rectum with steady pressure, then slowly squeeze the bottle until nearly all liquid is expelled. Insertion may be easier if person receiving enema bears down, as if having a bowel movement. This helps relax the muscles around the anus.
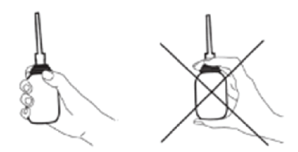
- Discontinue use if resistance is encountered. Forcing the enema can result in injury.
- Gently remove the tip while keeping the bottle compressed in order to prevent black-flow.
- Try to retain the solution for approximately 5-10 minutes or until you have a strong urge to defecate, so that it has time to exert its effect.
Stay near a toilet because most people have a bowel movement within 5-20 minutes. - Throw away the used bottle.
It is important to use KLYX Enema exactly as your doctor, nurse or pharmacist has described. KLYX should be used at times of the day as directed by your doctor, or at times that are best suited for your situation.
If you are using KLYX prior to a medical procedure, administration in the evening before the procedure and again on the day of your procedure, about 1 to 2 hours before your procedure, may be suitable.
Using it more often than recommended may not improve your problem any faster but may cause side effects, or increase the intensity of side effects.
Seek medical advice if symptoms persist.
If you are not sure how long to use KLYX Enema, talk to your doctor, nurse or pharmacist.
If you use too much (overdose)
If you may have administered too much KLYX Enema, immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital.
You may need urgent medical attention.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well after using KLYX.
This medicine helps most people who require a bowel preparation but it may have unwanted side effects in some people. All medicines can have side effects. Sometimes they are serious, most of the time they are not.
You may need medical attention if you get some of the following side effects:
- pain when pinching the rectum with the tip
- abdominal pain & discomfort
- effects from imbalance in the amount of salt in the body, such as irregular heartbeat, tiredness, nausea, vomiting, diarrhoea, convulsions
- dizziness or light-headedness, which may be due to low blood pressure
- slow heart beat (bradycardia).
Rare: Isolated cases of skin-rash and more severe types of allergy, with fall in blood-pressure and difficulty in breathing, have been reported.
If you notice any side effects not mentioned in this leaflet, please inform your doctor or pharmacist.
After using KLYX Enema
Storage
Keep your medicine in the pack until it is time to use it.
Keep KLYX Enema in a cool dry place where the temperature stays below 25°C. Do not refrigerate, burn or pierce KLYX.
Heat and dampness can destroy some medicines.
Keep it where children cannot reach it.
A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Do not use KLYX Enema after the expiry date stated on the label.
If it has expired or is damaged, return it to your pharmacist for disposal.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
KLYX Enema is supplied in a plastic bottle, of nominal volume 120 mL with a plastic applicator. The applicator is covered with a cap.
Ingredients
Your KLYX Enema contains:
Active Ingredients:
- docusate sodium 1 mg/mL and
- sorbitol solution (70 per cent) (crystallising) 357 mg/mL.
Inactive Ingredients:
- sodium methyl hydroxybenzoate (as a preservative)
- sodium propyl hydroxybenzoate (as a preservative)
- sodium hydroxide
- hydrochloric acid
- water-purified.
Sponsor
KLYX is supplied in Australia by:
Ferring Pharmaceuticals Pty Ltd
Suite 2, Level 1, Building 1
20 Bridge Street
Pymble NSW 2073
Australia
AUST R 287769 - KLYX 120 mL Enema
This leaflet was revised in March 2017. #38851-v3A
KLYX® is a registered trademark of Ferring B.V.
® = Registered trademark






