What is in this leaflet
This leaflet answers some common questions about Lescol XL.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
The information in this leaflet was last updated on the date listed on the final page. More recent information on the medicine may be available.
You should ensure that you speak to your pharmacist or doctor to obtain the most up to date information on the medicine. You can also download the most up to date leaflet from www.novartis.com.au. Those updates may contain important information about the medicine and its use of which you should be aware.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking Lescol XL against the benefits they expect it will provide.
If you have any concerns about this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What Lescol XL is used for
Lescol XL is used to lower the amount of cholesterol made by the body. Everyone has cholesterol in their blood. It is a type of fat needed by the body for many things, such as building cells, making bile acids (which help to digest food) and making some hormones. However, having too much cholesterol in the blood can contribute to the development of heart disease.
Cholesterol is present in many foods and is also made by your body. If your body does not balance the amount of cholesterol it makes with the amount of cholesterol you eat, then your cholesterol becomes too high. High cholesterol is more likely to occur with certain diseases or if you have a family history of high cholesterol.
Lescol XL does not reduce the cholesterol that comes from fat in food. Therefore, you also need to follow a low fat diet, control your weight and exercise regularly.
Lescol XL can also be used to help prevent further serious cardiac problems in people with heart disease who have already had cardiac catheterisation to open up the blood vessels in their heart.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
This medicine is only available with a doctor's prescription. It is not addictive.
There is not enough information to recommend this medicine for children.
Before you take Lescol XL
When you must not take it
Do not take Lescol XL if you have ever had an allergic reaction to:
- fluvastatin, the active ingredient in Lescol XL
- any of the other ingredients of Lescol XL listed at the end of this leaflet.
- other brands of fluvastatin
- other similar "statin" cholesterol lowering medicines (e.g. atorvastatin, pravastatin, simvastatin )
Some of the symptoms of an allergic reaction may include shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue or other parts of the body; rash, itching or hives on the skin.
Do not take Lescol XL if you are pregnant. It may affect your developing baby. If you think there is a possibility that you might become pregnant while you are taking this medicine, your doctor can give you advice about effective contraception.
Do not breast-feed while you are taking Lescol XL. It is not known if the active ingredient passes into the breast milk and could affect your baby.
Do not take Lescol XL after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. In that case, return it to your pharmacist.
Before you start to take it
Tell your doctor if you have any of the following health problems / medical conditions:
- a personal or family history of hereditary muscle disorders
- a personal or family history of diabetes
- a past history of inflammation of your muscles when you took a "statin" or "fibrate" medicine to lower your cholesterol. You may have had muscle pain, tenderness or weakness and unusual tiredness or fever.
- unexplained muscle pain, tenderness or weakness. These might be early signs of potentially severe muscle degradation.
- liver or kidney problems
- an underactive thyroid
- you regularly drink large amounts of alcohol
- a serious infection
- very low blood pressure (signs may include dizziness, light-headedness)
- recently had an injury
- severe metabolic, endocrine or electrolyte disorders such as decompensated diabetes and low blood potassium
- uncontrolled epilepsy, or
- if you are about to have an operation
Your doctor may not want you to take Lescol XL or will monitor you carefully to help prevent side effects during your treatment.
Tell your doctor if you are allergic to any other medicines, foods, dyes or preservatives. Your doctor will want to know if you are prone to allergies.
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you buy without a prescription from a pharmacy, supermarket or health food shop.
Some medicines and Lescol XL may interfere with each other. These include:
- other cholesterol lowering medicines, including colestyramine, gemfibrozil and nicotinic acid
- warfarin, a medicine to prevent blood clots
- some antibiotics, including erythromycin, fusidic acid and itraconazole
- cimetidine, ranitidine and omeprazole, medicines used to treat stomach ulcers and reflux
- ciclosporin
- phenytoin, a medicine used to treat epilepsy
- rifampicin, a medicine used to treat tuberculosis
You may need to take different amounts of your medicines or to take different medicines while you are taking Lescol XL. Your doctor and pharmacist have more information.
If you have not told your doctor about any of these things, tell him/her before you start taking this medicine.
How to take Lescol XL
Follow all directions given to you by your doctor and pharmacist carefully. These instructions may differ from the information contained in this leaflet.
If you do not understand the instructions on the label, ask your doctor or pharmacist for help.
How much to take
Lescol XL prolonged released tablet is available in 80mg.
To prevent complications in people with heart disease, the usual daily dose is 80 mg.
How to take it
Take the capsules with a full glass of water.
Lescol XL must not be chewed, crushed, cut in half or split.
Lescol XL can be taken anytime of the day.
If you are also taking a medicine called colestyramine to help lower your cholesterol, take your dose of Lescol XL at least 4 hours after taking the dose of colestyramine. These two medicines will interact with one another if they are taken too close together.
Take Lescol XL at about the same time each day. Taking your medicine at the same time each day will have the best effect. It will also help you remember when to take it.
How long to take it
Take this medicine for as long as your doctor recommends.
Lescol XL helps to lower your cholesterol but does not cure your condition. You must continue to take it as directed to keep your cholesterol levels down.
If you forget to take it
If your next dose is not due for more than 4 hours, take the missed dose as soon as you remember. Then take your next dose at the usual time and continue on with your normal schedule.
But, if your next dose is due in less than 4 hours, skip the missed dose. Take your next dose at the usual time and continue on with your normal schedule.
Do not take a double dose to make up for the one that you missed. This may increase the chance of you getting an unwanted side effect.
If you have trouble remembering when to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or Poisons Information Centre (telephone number 13 11 26), or go to Accident and Emergency at your nearest hospital if you think that you or anyone else may have taken too much Lescol XL. Do this even if there are no signs of discomfort or poisoning. Keep the telephone numbers for these places handy.
While you are taking Lescol XL
Things you must do
If you become pregnant while taking Lescol XL, stop taking it and tell your doctor immediately.
Continue on your cholesterol lowering diet while you are taking Lescol XL.
Have your cholesterol levels checked and any other blood tests done as directed by your doctor. Regular blood tests will help to make sure the treatment is working and prevent unwanted side effects.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking Lescol XL.
Tell any other doctor, dentist or pharmacist who treats you that you are taking Lescol XL.
Things you must not do
Do not give this medicine to anyone else, even if their condition seems similar to yours.
Do not take it to treat any other complaints unless your doctor tells you to.
Things to be careful of
Be careful driving or operating machinery until you know how Lescol XL affects you. There is no information on the possible effects of this medicine on mental alertness. Make sure you know how you react to it before you drive a car, operate machinery or do anything else that could be dangerous.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Lescol XL, even if you do not think it is connected with the medicine.
All medicines can have side effects. Sometimes they are serious, but most of the time they are not. You may need medical treatment if you get some of the side effects.
Do not be alarmed by these lists of possible side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you have signs and symptoms of high levels of blood sugar such as an excessive thirst, high urine output, increased appetite with weight loss, tiredness.
Tell your doctor if you notice any of the following and they worry you:
- upset stomach or heartburn
- nausea (feeling sick)
- diarrhoea
- headache
- difficulty sleeping
- numbness or tingling in the hands or feet
- prickling or burning sensation or decreased sensitivity of the skin
- impotence
- unexplained muscle pain, tenderness or weakness
Tell your doctor immediately or go to Accident and Emergency at your nearest hospital if you notice any of the following:
- unexpected pain, tenderness or weakness in your muscles, which may be accompanied by unusual tiredness or fever. These may be early signs of an inflammation of your muscles that can lead to destruction of muscle tissue. This can be avoided if your doctor stops your treatment as quickly as possible.
- signs of allergy such as swelling of the face, eyelids, lips, tongue or other parts of the body; rash, itching or hives on the skin; shortness of breath, wheezing or difficulty breathing; unusual bleeding or bruising under the skin.
- signs of a possible liver problem, including yellowing of the skin and/or eyes and dark coloured urine, nausea, vomiting, loss of appetite, impaired brain function, easy bruising or bleeding.
- signs of a possible lung problem, including shortness of breath, non-productive cough, fatigue, weight loss and fever.
Tell your doctor if you notice anything else that is making you feel unwell. Some people may have other side effects not yet known or mentioned in this leaflet.
After using Lescol XL
Storage
- Keep your medicine in the original container until it is time to take it.
- Store it in a cool dry place.
- Do not store Lescol XL or any other medicine in the bathroom or near a sink.
- Do not leave it in the car or on window sills.
Keep the medicine where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking Lescol XL or the expiry date has passed, ask your pharmacist what to do with any medicine you have left over.
Product description
What it looks like
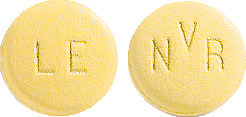
Lescol XL 80 mg tablet: yellow, round, slightly biconvex film-coated tablet, marked "LE" on one side and "NVR" on the other; packs of 28.
Ingredients
Lescol XL tablets contain 80 mg of the active ingredient, fluvastatin (as the sodium salt).
They also contain:
- magnesium stearate
- potassium bicarbonate
- hypromellose
- microcrystalline cellulose
- hyprolose
- povidone
- yellow film coating (containing macrogol, titanium oxide, iron oxide yellow)
Lescol XL does not contain sucrose, gluten, tartrazine or any other azo dyes.
Sponsor
Lescol XL is supplied in Australia by:
NOVARTIS Pharmaceuticals Australia Pty Limited
ABN 18 004 244 160
54 Waterloo Road
Macquarie Park NSW 2113
Telephone: 1 800 671 203
®= Registered Trademark
This leaflet was prepared in
January 2021
Australian Registration Number.
Lescol XL 80mg AUSTR 82743
(les290121c.doc) based on PI (les290121i.doc)
Published by MIMS March 2021



 LIPS indicated that, after a median of 3.9 years of treatment, there was a statistically significant reduction in the proportion of patients who had suffered a first MACE. In the fluvastatin group, 78.3% of patients survived 4 years without suffering a MACE compared to 72.6% in the placebo group. There was no statistically significant separation of the effect between fluvastatin and placebo until 3 years. Prescribers should note that the benefit of fluvastatin treatment for periods of less than 4.5 years was not examined in the LIPS trial.
LIPS indicated that, after a median of 3.9 years of treatment, there was a statistically significant reduction in the proportion of patients who had suffered a first MACE. In the fluvastatin group, 78.3% of patients survived 4 years without suffering a MACE compared to 72.6% in the placebo group. There was no statistically significant separation of the effect between fluvastatin and placebo until 3 years. Prescribers should note that the benefit of fluvastatin treatment for periods of less than 4.5 years was not examined in the LIPS trial. In these studies, both fluvastatin 40 mg twice a day and Lescol XL 80 mg once daily reduced total-C, LDL-C, apolipoprotein-B (Apo-B) and triglycerides (TG), and increased HDL-C after 24 weeks of therapy (see Table 3).
In these studies, both fluvastatin 40 mg twice a day and Lescol XL 80 mg once daily reduced total-C, LDL-C, apolipoprotein-B (Apo-B) and triglycerides (TG), and increased HDL-C after 24 weeks of therapy (see Table 3). Of the 857 patients randomised to Lescol XL 80 mg, 271 with primary mixed dyslipidaemia (Fredrickson type IIb) as defined by baseline plasma triglyceride levels ≥ 2.25 mmol/L (200 mg/dL), had a median reduction in triglycerides of 25%. In these patients, Lescol XL 80 mg produced meaningful increases in HDL-C of 13%. This effect was even more pronounced in those patients with very low HDL-C levels at baseline (i.e. ≤ 0.9 mmol/L (35 mg/dL)), who had mean increases in HDL-C of 16%. Significant decreases in total-C, LDL-C and Apo-B were also achieved. In these studies, patients with triglycerides > 4.5 mmol/L (400 mg/dL) were excluded.
Of the 857 patients randomised to Lescol XL 80 mg, 271 with primary mixed dyslipidaemia (Fredrickson type IIb) as defined by baseline plasma triglyceride levels ≥ 2.25 mmol/L (200 mg/dL), had a median reduction in triglycerides of 25%. In these patients, Lescol XL 80 mg produced meaningful increases in HDL-C of 13%. This effect was even more pronounced in those patients with very low HDL-C levels at baseline (i.e. ≤ 0.9 mmol/L (35 mg/dL)), who had mean increases in HDL-C of 16%. Significant decreases in total-C, LDL-C and Apo-B were also achieved. In these studies, patients with triglycerides > 4.5 mmol/L (400 mg/dL) were excluded. The second study (B2301) enrolled 85 male and female patients, 10 to 16 years of age, who had an LDL-C ≥ 4.9 mmol/L (190 mg/dL) or LDL-C ≥ 4.1 mmol/L (160 mg/dL) and one or more risk factors for coronary heart disease, or LDL-C > 4.1 mmol/L (160 mg/dL) and a proven LDL-receptor defect. The main exclusion criteria were patients with homozygous familial hypercholesterolaemia; secondary forms of dyslipoproteinaemia; serum triglyceride levels > 6.8 mmol/L (600 mg/dL); ALAT, ASAT or creatinine levels > 1.5 x ULN; serum CK or serum TSH > 2 x ULN; BMI > 30 kg/m2. All patients were started on fluvastatin 20 mg daily with dose adjustments every 6 weeks to 40 mg daily then 80 mg daily (Lescol 80 mg XL tablet) to achieve an LDL-C goal of ≤ 3.4 mmol/L (130 mg/dL). 70 patients were pubertal or postpubertal (n = 69 evaluated for efficacy; see Table 4). The mean baseline LDL-C was 5.8 mmol/L (225 mg/dL) (range 3.8 to 8.9 mmol/L).
The second study (B2301) enrolled 85 male and female patients, 10 to 16 years of age, who had an LDL-C ≥ 4.9 mmol/L (190 mg/dL) or LDL-C ≥ 4.1 mmol/L (160 mg/dL) and one or more risk factors for coronary heart disease, or LDL-C > 4.1 mmol/L (160 mg/dL) and a proven LDL-receptor defect. The main exclusion criteria were patients with homozygous familial hypercholesterolaemia; secondary forms of dyslipoproteinaemia; serum triglyceride levels > 6.8 mmol/L (600 mg/dL); ALAT, ASAT or creatinine levels > 1.5 x ULN; serum CK or serum TSH > 2 x ULN; BMI > 30 kg/m2. All patients were started on fluvastatin 20 mg daily with dose adjustments every 6 weeks to 40 mg daily then 80 mg daily (Lescol 80 mg XL tablet) to achieve an LDL-C goal of ≤ 3.4 mmol/L (130 mg/dL). 70 patients were pubertal or postpubertal (n = 69 evaluated for efficacy; see Table 4). The mean baseline LDL-C was 5.8 mmol/L (225 mg/dL) (range 3.8 to 8.9 mmol/L). Empirical formula: C24H25FNO4.Na.
Empirical formula: C24H25FNO4.Na.