What is in this leaflet
This leaflet answers some common questions about Lioresal.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
The information in this leaflet was last updated on the date listed on the final page. More recent information on the medicine may be available.
You should ensure that you speak to your pharmacist or doctor to obtain the most up to date information on the medicine. You can also download the most up to date leaflet from www.novartis.com.au.
Those updates may contain important information about the medicine and its use of which you should be aware.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking Lioresal against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What Lioresal is used for
Lioresal belongs to a group of medicines called muscle relaxants. This medicine is used to reduce the excessive stiffness and/or spasms in your muscles. These spasms happen in various illnesses such as multiple sclerosis and diseases or injuries of the spinal cord.
Because this medicine reduces spasms and the pain that goes with them, it helps to make you more mobile and better able to manage your daily activities.
Ask your doctor if you have any questions about why Lioresal has been prescribed for you. Your doctor may have prescribed it for another purpose.
Lioresal is only available with a doctor's prescription.
Before you take Lioresal
When you must not take it
Do not take Lioresal if you have ever had an allergic reaction after taking:
- baclofen (the active ingredient in Lioresal)
- any of the other ingredients listed at the end of this leaflet
Some of the symptoms of an allergic reaction may include shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue or other parts of the body; rash, itching or hives on the skin.
Do not take Lioresal after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. In that case, return it to your pharmacist.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you are pregnant or intend to become pregnant or you are breast-feeding. There is very little information on the use of this medicine in pregnancy or while breast-feeding. Your doctor can discuss with you the risks and benefits involved.
If you have to take Lioresal during pregnancy your baby may have convulsions and other symptoms related to sudden discontinuation of the medicine just after delivery.
Tell your doctor if you have, or have ever had, any of the following medical conditions:
- a mental illness
- Parkinson's disease
- seizures (fits) from any cause
- stiffness and restriction of movement in a group of muscles
- stomach ulcers
- stroke or other blood circulation disease
- heart disease
- kidney disease
- liver disease
- lung problems which make breathing difficult
- diabetes
- porphyria, a rare disturbance in the production of porphyrin, a pigment important for liver function and blood formation
- history of alcoholism, drink alcohol to excess or you have a history of drug abuse or dependence. Some people being treated with baclofen have had thoughts of harming or killing themselves or have tried to kill themselves. Most of these people also had depression, had been using alcohol excessively or were prone to having thoughts of killing themselves. If you have thoughts of harming or killing yourself at any time, speak to your doctor straightaway or go to a hospital. Also, ask a relative or close friend to tell you if they are worried about any changes in your behaviour and ask them to read this leaflet.
- high blood pressure
Your doctor may want to take special precautions if you have any of the above conditions.
These precautions may include additional tests during or prior to taking Lioresal.
If you have not told your doctor about any of these things, tell him/her before you take Lioresal.
Taking other medicines
Tell your doctor if you are taking any other medicines, including medicines that you buy without a prescription from a pharmacy, supermarket or health food shop.
Some medicines and Lioresal may interfere with each other. These include:
- any medicine that tends to make you sleepy, such as medicines used to help you sleep or calm you down, pain relievers and medicines for colds or allergies
- medicines used to treat mood disorders, including tricyclic antidepressants, lithium and monoamine oxidase inhibitors (MAOIs)
- medicines used to treat diabetes
- medicines for high blood pressure
- medicines used to treat Parkinson's disease, including selegiline and levodopa and carbidopa
You may need to take different amounts of your medicines or you may need to take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking Lioresal.
Tell your doctor if you have an intolerance to gluten. This medicine contains wheat starch.
How to take Lioresal
Follow all directions given to you by your doctor and pharmacist carefully. These directions may differ from the information contained in this leaflet.
If you do not understand the instructions on the label, ask your doctor or pharmacist for help.
How much to take
Treatment is usually started in hospital with small doses of Lioresal. The dose is then gradually increased to an amount that works best for you. For example, Lioresal may be started at a dose of 15 mg a day, then increased slowly to anywhere from 30 to 75 mg a day. Sometimes, doses up to 100 mg a day may be needed.
If you are under the age of 16 or over 65 or you have kidney disease, your doctor may start you on a lower dose and increase it more gradually to prevent unwanted side effects.
How to take it
Swallow the tablets during meals with a little liquid.
Lioresal is usually taken in at least 3 divided doses throughout the day. But your doctor may tell you to take it more or less often, depending on your situation.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and take the next dose when you are meant to.
Otherwise, take it as soon as you remember and then go back to taking it as you would normally.
Do not take a double dose to make up for the one that you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering when to take your medicine, ask your pharmacist for some hints.
How long to take it
Continue taking Lioresal for as long as your doctor recommends. Your doctor will check your progress to make sure the medicine is working and will discuss with you how long your treatment should continue.
If you take too much (Overdose)
Immediately telephone your doctor or Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at your nearest hospital if you think that you may have taken too much Lioresal, even if there are no signs of discomfort or poisoning.
The main symptoms of overdose are: drowsiness, breathing difficulties, consciousness disorders and being unconscious (coma).
Other symptoms may include: feeling confused, hallucinations, agitation, convulsions, blurred vision, unusual muscle slackness, sudden contraction of the muscles, poor or absent reflexes, high or low blood pressure, slow, fast or irregular heartbeat, low body temperature, nausea, vomiting, diarrhoea or excessive salivation, trouble breathing during sleep (sleep apnoea), pain in muscles, fever and dark urine (rhabdomyolysis).
If you have kidney disease and have accidentally taken more tablets or more syrup than your doctor has prescribed, you may experience neurological symptoms of overdose (e.g. drowsiness, feeling confused, hallucinations)
While you are taking Lioresal
Things you must do
If you become pregnant while taking Lioresal, tell your doctor immediately. Your doctor can discuss with you the risks of taking it while you are pregnant.
Be sure to keep all of your doctor's appointments so that your progress can be checked. To help prevent unwanted side effects from happening, your doctor may want to do some tests from time to time during the course of your treatment.
If your muscle spasms come back, tell your doctor. Your doctor may be able to change the dose of Lioresal to make it work better for you.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking Lioresal.
Tell any other doctor, dentist or pharmacist who treats you that you are taking Lioresal.
Things you must not do
Do not stop taking Lioresal suddenly. This medicine is not habit-forming but stopping it suddenly may bring on severe spasms and other unwanted symptoms, such as nervousness, feeling confused, hallucinations, abnormal thinking or behaviour, convulsions, uncontrollable twitching, jerking or writhing movements, fast heartbeat, high body temperature, pain in muscles, fever and dark urine. The excessive stiffness (spasms) in your muscles may also get worse.
If Lioresal must be stopped, your doctor will reduce the dose gradually over a period of 1 to 2 weeks so that these unwanted effects are avoided.
Do not use Lioresal to treat any other complaints unless your doctor tells you to.
Do not give this medicine to anyone else, even if their symptoms seem to be similar to yours.
Things to be careful of
Be careful driving, operating machinery or doing jobs that require you to be alert while you are taking Lioresal until you know how it affects you. This medicine may cause sleepiness and decreased alertness in some people, especially at the start of treatment.
Be careful when drinking alcohol while you are taking Lioresal. The combination may make you feel more sleepy and less alert than usual.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Lioresal.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Side effects happen mainly at the start of treatment or if the dose is too high or is increased too rapidly. They can often be relieved by lowering the dose.
If you are over 65 years old, you should be especially careful while taking this medicine. Report any side effects promptly to your doctor. As people grow older, they are more likely to get side effects from medicines.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you notice any of the following and they worry you:
- daytime sleepiness or drowsiness
- lack of energy, tiredness
- dizziness or light-headedness
- spinning sensation (vertigo)
- mental confusion
- headache
- difficulty sleeping or nightmares
- nausea (feeling sick), retching or vomiting
- constipation, stomach cramps or diarrhoea
- loss of appetite
- stuffy or blocked nose
- dry mouth
- change in sense of taste
- misuse, abuse and dependence
- suicide or suicide attempts
- numbness or tingling in hands and feet
- muscle weakness, spasms or pain
- problems with coordination and balance
- difficulty in speaking
- swelling of ankles due to fluid build-up
- blurred or double vision
- ringing in the ears
- frequent urination or bed wetting
- excessive sweating
- weight gain
- impotence or inability to ejaculate
- increased blood sugar
- low body temperature
Tell your doctor immediately or go to Accident and Emergency at your nearest hospital if you notice any of the following:
- signs of allergy such as rash, itching or hives on the skin; swelling of the face, lips, tongue or other parts of the body; shortness of breath or wheezing
- slow or difficult breathing
- irregular heart beat (fast or slow)
- chest pain
- uncontrollable muscle spasms affecting the eyes, head, neck or body
- fainting or seizures (fits)
- depression or other severe mood or mental changes
- hallucinations (hearing or seeing things that are not there)
- being unable to urinate or pain when urinating; blood in the urine
- symptoms following sudden discontinuation of the medicine (drug withdrawal syndrome). Refer to "Things you must not do" above
The above side effects could be serious. You may need medical attention.
Tell your doctor if you notice anything else that is making you feel unwell. Other side effects not listed above may happen in some people.
After using Lioresal
Storage
- Keep your tablets in the original container until it is time to take them
- Store the tablets in a cool dry place
- Do not store Lioresal or any other medicine in the bathroom or near a sink
- Do not leave it in the car or on window sills.
Heat and dampness can destroy some medicines. Lioresal will keep well if it is cool and dry.
Keep the tablets where young children cannot reach them. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking Lioresal or the expiry date on the medicine has passed, ask your pharmacist what to do with any tablets you have left over.
Product description
What it looks like
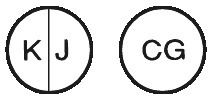
Lioresal 10 mg: white, round, flat tablets marked with KJ and a score line on one side and CG on the other side: packs of 100 tablets.
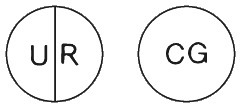
Lioresal 25 mg: white, round, flat tablets marked with UR and a score line on one side and CG on the other side: packs of 100 tablets.
Ingredients
Lioresal tablets contain either 10 mg or 25 mg of baclofen, the active ingredient. They also contain:
- colloidal anhydrous silica
- microcrystalline cellulose
- magnesium stearate
- povidone
- wheat starch
Lioresal tablets do not contain lactose, sucrose, tartrazine or any other azo dyes.
Excipient with known effect: wheat starch. Wheat starch may contain gluten, but only in trace amounts.
Sponsor
Lioresal is supplied in Australia by:
NOVARTIS Pharmaceuticals
Australia Pty Limited
ABN 18 004 244 160
54 Waterloo Road
Macquarie Park NSW 2113
Telephone 1 800 671 203
®= Registered Trademark
This leaflet was prepared in November 2019.
Australian Registration Number.
Lioresal 10 mg bottle: AUST R 11038
Lioresal 25 mg bottle: AUST R 11039
Lioresal 25 mg blister: AUST R 68365
Internal document code
(lrst071119c) based on PI (lrst071119i)
Published by MIMS January 2020