What is in this leaflet
This leaflet answers some common questions about Livial. It does not contain all the available information.
It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking Livial against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this information with the pack. You may wish to read it again.
What Livial is used for
Livial tablets contain the active ingredient tibolone, which is a synthetic steroid medicine used for hormone replacement therapy (HRT). It mimics the activity of the female sex hormones in the body.
Livial contains tibolone, a substance that has favourable effects on different tissues in the body, such as brain, vagina and bone. Livial is used in postmenopausal women at least 12 months since their last natural period.
Livial is used for:
Relief of symptoms occurring after menopause
During the menopause, the amount of estrogen produced by a woman's body drops. This can cause symptoms such as hot face, neck and chest ("hot flushes"). Livial alleviates these symptoms after menopause. You will only be prescribed Livial if your symptoms seriously hinder your daily life.
Prevention of osteoporosis
After the menopause some women may develop fragile bones (osteoporosis). You should discuss all available options with your doctor.
If you are at an increased risk of fractures due to osteoporosis and other medicines are not suitable for you, you can take Livial to prevent osteoporosis after menopause.
Livial is not a contraceptive.
Livial has no effect on alertness and concentration as far as is known.
A doctor's prescription is required to obtain this medicine.
Before you use Livial
When you must not take it
Do not take Livial if:
- you are pregnant or think you may be pregnant
- you are breastfeeding
- you have or have ever had breast cancer, or if you are suspected of having it
- you have cancer which is sensitive to estrogens, such as cancer of the womb lining (endometrium), or if you are suspected of having it
- you have any unexplained vaginal bleeding
- you have excessive thickening of the womb lining (endometrial hyperplasia) that is not being treated
- you have or have ever had a blood clot in a vein (thrombosis), such as in the legs (deep venous thrombosis) or the lungs (pulmonary embolism)
- you have a blood clotting disorder (such as protein C, protein S, or antithrombin deficiency)
- you have or recently have had a disease caused by blood clots in the arteries, such as a heart attack, stroke or angina
- you have or have ever had liver disease and your liver function tests have not returned to normal
- you have a rare blood problem called porphyria which is passed down in families (inherited)
- you are allergic (hypersensitive) to tibolone or any of the ingredients of Livial listed at the end of this leaflet
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or troubled breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin.
If any of the above conditions appear for the first time while taking Livial, stop taking it at once and consult your doctor immediately.
Medical history and regular check-ups
As well as benefits, HRT or Livial has some risks which need to be considered when deciding whether to starting taking it, or whether to carry on taking it.
The experience in treating women with a premature menopause (due to ovarian failure or surgery) is limited. If you have a premature menopause the risks of using HRT or Livial may be different. Please talk to your doctor.
Before you start (or restart) HRT or Livial, your doctor will ask you about your own and your family's medical history. Your doctor may decide to perform a physical examination. This may include an examination of your breasts and/or an internal examination, if necessary.
Once you have started on Livial, you should see your doctor for regular check-ups (at least once a year). At these check-ups, discuss with your doctor the benefits and risks of continuing with Livial.
Go for regular breast screening, as recommended by your doctor.
When to take special care with Livial
Tell your doctor if you have ever had any of the following conditions before you start the treatment, as these may return or become worse during treatment with Livial. If so, you should see your doctor more often for check-ups:
- fibroids inside your womb
- growth of the womb lining outside your womb (endometriosis) or a history of excessive growth of the womb lining (endometrial hyperplasia)
- increased risk of developing blood clots (see "Blood clots in a vein (thrombosis)")
- increased risk of getting an estrogen-sensitive cancer (such as having a mother, sister or grandmother who has had breast cancer)
- high blood pressure
- a liver disorder, such as a benign liver tumour
- diabetes
- gallstones
- migraine or severe headaches
- a disease of the immune system that affects many organs of the body (systemic lupus erythematosus, SLE)
- epilepsy
- asthma
- a disease affecting the eardrum and hearing (otosclerosis)
- a very high level of fat in your blood (triglycerides)
- fluid retention due to cardiac or kidney problems
Tell your doctor if you notice any change in your condition whilst using Livial.
Stop taking Livial and see a doctor immediately if you notice any of the following when taking HRT or Livial:
- any of the conditions mentioned in the "Do not take Livial" section
- yellowing of your skin or the whites of your eyes (jaundice). These may be signs of a liver disease
- a large rise in your blood pressure (symptoms may be headache, tiredness, dizziness)
- migraine-like headaches which happen for the first time
- if you become pregnant
- if you notice signs of a blood clot, such as:
- painful swelling and redness of the legs
- sudden chest pain
- difficulty in breathing
For more information, see "Blood clots in a vein (thrombosis)"
HRT and cancer
Excessive thickening of the lining of the womb (endometrial hyperplasia) and cancer of the lining of the womb (endometrial cancer)
There have been reports and studies of an increased cell growth or cancer of the lining of the womb in women using Livial. The risk of cancer of the lining of the womb increases with the duration of use.
Irregular bleeding
You may have irregular bleeding or drops of blood (spotting) during the first 3-6 months of taking Livial.
However, if the irregular bleeding:
- carries on for more than the first 6 months
- starts after you have been taking Livial for more than 6 months
- carries on after you have stopped taking Livial
see your doctor as soon as possible.
Breast cancer
Taking estrogen, estrogen-progesterone combined HRT or Livial for several years increases the risk of breast cancer. The risk increases with the duration of HRT use and returns to normal within about 5 years after stopping HRT.
Women taking Livial have a lower risk than women using combined HRT and a comparable risk with estrogen-only HRT.
Regularly check your breasts. See your doctor if you notice any changes such as:
- dimpling or sinking of the skin
- changes in the nipple
- any lumps you can see or feel
Ovarian cancer
Ovarian cancer is rare. An increased risk of ovarian cancer has been reported in women taking HRT after 5 years of use.
For women aged 50 to 54 who are not taking HRT, about 2 women in 2000 will be diagnosed with ovarian cancer over a 5-year period. For women who have been taking HRT for 5 years, there will be 1 extra case per 2000 users. With use of Livial, the increased risk of ovarian cancer for women taking Livial for 5 years is 1 extra case per 2500 users.
Effects of HRT on heart or circulation
Heart disease (heart attack)
There is no evidence that HRT or Livial will prevent a heart attack.
Women over the age of 60 who use estrogen-progestogen HRT are slightly more likely to develop heart disease than those not taking any HRT. As the risk of heart disease strongly depends on age, the number of extra cases of heart disease due to use of estrogen-progestogen HRT is very low in healthy women close to menopause, but will rise with more advanced age.
There is no evidence to suggest that the risk of myocardial infarction with Livial is different to the risk of other HRT.
See a doctor as soon as possible and do not take any more Livial if you get a pain in your chest that spreads to your arm or neck. This pain could be a sign of heart disease.
Stroke
Recent research suggests that HRT and Livial increases the risk of having a stroke. This increased risk has mainly been observed in elderly postmenopausal women above 60 years of age.
Looking at women in their 50s who are not taking Livial - on average, over a 5-year period, 3 in 1000 would be expected to have a stroke. For women in their 50s who are taking Livial, the figure would be 7 in 1000 (i.e. an extra 4 cases).
Looking at women in their 60s who are not taking Livial - on average, over a 5-year period, 11 in 1000 would be expected to have a stroke. For women in their 60s who are taking Livial, the figure would be 24 in 1000 (i.e. an extra 13 cases).
If you are worried about any of these things, or if you have had a stroke in the past, talk to your doctor to see if you should take Livial.
See a doctor as soon as possible and do not take any more Livial until your doctor says you can if you get any unexplained migraine-type headaches with or without disturbed vision. These headaches may be an early warning sign of a stroke.
Blood clots in a vein (Thrombosis)
Estrogen and estrogen-progestogen combined HRT may increase the risk of blood clots in the veins (also called deep vein thrombosis, or DVT), especially during the 1st year of taking it. It is unknown if Livial increases the risk in the same way.
Blood clots can be serious, and if one travels to the lungs, it can cause chest pain, breathlessness, fainting or even death. You are more likely to get a blood clot in your veins as you get older and if any of the following applies to you. Inform your doctor if any of these situations apply to you:
- you are pregnant or recently had a baby
- you use estrogens
- you are unable to walk for a long time because of major surgery, injury or illness (see also "If you need to have surgery")
- you are seriously overweight (BMI greater than 30kg per square metre)
- you have any blood clotting problem that needs long-term treatment with a medicine used to prevent blood clots
- if any of your close relatives has ever had a blood clot in the leg, lung or another organ
- you have systemic lupus erythematosus (SLE, a disease of your immune system)
- you have cancer
If any of these apply to you, talk to your doctor about whether you should use Livial.
For signs of a blood clot, see "Stop taking Livial and see a doctor immediately".
Looking at women in their 50s who are not taking HRT, on average, over a 5-year period, 4 to 7 in 1000 would be expected to get a blood clot in a vein.
For women in their 50s who have been taking estrogen-progestogen HRT for over 5 years, there will be 9 to 12 cases in 1000 users (i.e. an extra 5 cases).
With use of Livial, the increased risk of getting a blood clot in a vein is lower than with other types of HRT.
Other conditions
HRT will not prevent memory loss. There is some evidence of a higher risk of memory loss in women who start using HRT after the age of 65. Speak to your doctor for advice.
Tell your doctor if you become pregnant or are breast-feeding. Livial is for use in postmenopausal women only. If you are pregnant or breast-feeding or think you may be pregnant, do not take Livial.
Tell your doctor if you react badly to lactose or milk before you start taking Livial. Livial tablets contain lactose.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including medicines that you get without a prescription, herbal medicines or other natural products from your pharmacy, supermarket or health food shop. Some medicines may interfere with the effect of Livial.
This applies to the following medicines:
- medicines against blood clotting (such as warfarin)
- medicines for epilepsy (such as phenobarbitone, phenytoin and carbamazepine)
- medicines for tuberculosis (such as rifampicin)
- herbal remedies containing St John's Wort (hypericum perforatum)
Your doctor may need to adjust the dose of these medicines.
Ask your doctor or pharmacist for advice before taking any medicine.
How to take Livial
When to start it
Livial should not be taken until 12 months after your last natural menstrual bleed. If Livial is taken sooner than this, the chance of irregular vaginal bleeding may be increased.
Women who have undergone premature menopause (surgical removal of ovaries) can start taking Livial immediately.
If you are already using a different type of HRT, your doctor will advise you when to switch to Livial.
How to take it
Take Livial as directed by your doctor. You should also read the instructions given in this leaflet for your medicine. If you are not sure how to take Livial ask your doctor or pharmacist.
Take one tablet daily, at about the same time each day. Swallow the tablet with some water or other non-alcoholic drink.
The Livial pack contains 28 white tablets. The strips with Livial are marked with the days of the week. Start by taking the tablet marked with that day. For example, if it is a Monday, take a tablet marked Monday on the upper row of the strip. Follow the days of the week until the strip is empty. Start the next strip the next day.
Do not leave a break between strips or packs.
How long to use it
HRT should be prescribed at the lowest effective dose and for the shortest duration necessary. Your doctor can advise you how long you may need to take Livial.
If you forget to take it
If you forget to take a tablet, take it as soon as you remember, unless you are more than 12 hours late. If you are more than 12 hours late, skip the dose you missed and take your next dose when you are meant to.
Do not take a double dose to make up for the forgotten dose.
If you take too much (overdose)
If you have taken more tablets than you have been prescribed, immediately telephone your doctor or the Poisons Information Centre on 13 11 26, for advice.
Signs of an overdose may include feeling sick or vomiting. Vaginal bleeding may also occur after a few days.
If you need to have surgery
Tell your doctor and your surgeon that you are taking Livial if you are going to have surgery.
You may need to stop taking Livial about 4-6 weeks before the operation to reduce the risk of a blood clot (see "Blood clots in a vein"). Ask your doctor when you can start taking Livial again.
Side Effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Livial.
The following diseases are reported more often in women using HRT compared to women not using HRT:
- breast cancer
- abnormal growth or cancer of the lining of the womb (endometrial hyperplasia or cancer)
- ovarian cancer
- blood clots in the veins of the legs or lungs (venous thromboembolism)
- heart disease
- stroke
- probable memory loss if HRT is started over the age of 65
Livial helps most women with menopausal symptoms, but it may have unwanted side effects in a few people. All medicines can have side effects. Sometimes they are serious, most of the time they are not.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Common side effects observed in clinical studies (occurring in 1-10% of the women using Livial) were:
- vaginal bleeding or spotting
- abdominal pain
- weight gain
- breast pain
- unnatural hair growth
- vaginal symptoms, such as discharge, itching, and irritation
Uncommon side effects (occurring in 0.1-1% of the women using Livial) were:
- acne
Other side effects observed with Livial in market use were:
- dizziness, headache, migraine, depression
- rash or itching
- visual disturbances
- gastro-intestinal upset
- fluid retention
- joint pain, muscle pain
- changes in liver function
There have been reports of breast cancer and of an increased cell growth or cancer of the lining of the womb in women using Livial.
Tell your doctor if vaginal bleeding or spotting occurs or if any of the above mentioned side effects worry you or continue.
Please see "Medical history and regular check-ups" for conditions where Livial should be stopped.
The following side effects have been reported with other HRTs:
- gall bladder disease
- various skin disorders:
- discolouration of the skin especially of the face or neck known as "pregnancy patches" (chloasma)
- painful reddish skin nodules (erythema nodosum)
- rash with target-shaped reddening or sores (erythema multiforme)
Tell your doctor or pharmacist if you notice any side effects not mentioned in this leaflet.
After Using Livial
Storage
Keep your Livial tablets in a safe place out of the reach of children.
Keep your Livial tablets in the original package in a cool dry place where the temperature stays below 25°C.
Do not use after the expiry date stated on the blister and outer box.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product Description
What it looks like
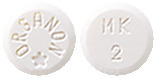
Packs contain one blister strip of 10 or 28 white round flat tablets with bevelled edges. The tablets are marked Organon and a star on one side and MK above 2 on the other.
Ingredients
Livial tablet contains 2.5 mg of the active ingredient called tibolone. Livial also contains the following inactive ingredients:
- potato starch
- lactose monohydrate
- ascorbyl palmitate
- magnesium stearate
Do not use the product if the blister pack or tablets are damaged or appear unusual.
Supplier
Organon Pharma Pty Ltd
Building A, 26 Talavera Rd,
Macquarie Park NSW 2113
Australia
AUST R 55088
This leaflet was prepared in
December 2020.
CCPPI-MK8471-TB-112012
Published by MIMS February 2021

 In market use, other undesirable effects that have been observed include dizziness, rash, pruritus, seborrheic dermatosis, headache, migraine, visual disturbances (including blurred vision), gastrointestinal upset, depression, oedema, effects on the musculoskeletal system such as arthralgia or myalgia and changes in liver function parameters.
In market use, other undesirable effects that have been observed include dizziness, rash, pruritus, seborrheic dermatosis, headache, migraine, visual disturbances (including blurred vision), gastrointestinal upset, depression, oedema, effects on the musculoskeletal system such as arthralgia or myalgia and changes in liver function parameters.

 Molecular formula: C21H28O2.
Molecular formula: C21H28O2.