1 Name of Medicine
Metoclopramide hydrochloride.
2 Qualitative and Quantitative Composition
Tablets.
Metoclopramide 10 mg (as hydrochloride).
Maxolon tablets contain lactose monohydrate. For the full list of excipients, see Section 6.1 List of Excipients.3 Pharmaceutical Form
Tablets.
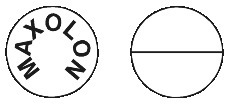
Round biconvex, white tablets approximately 7 mm in diameter with a break bar on one side and "MAXOLON" debossed on the other.4.1 Therapeutic Indications
Adults (20 years and over).
As an adjunct to X-ray examination of the stomach and duodenum.
To assist in intestinal intubation.
To control nausea and vomiting associated with the following conditions: intolerance to essential drugs possessing emetic properties; uraemia; radiation sickness; malignant disease; postoperative vomiting; labour; infectious diseases. There is no clear benefit in motion sickness or other labyrinth disturbances.
Maxolon has been found useful in the management of gastric retention after gastric surgery.
Maxolon may be useful in the treatment of diabetic gastroparesis of mild to moderate severity. Once control of diabetes has been established by diet and/or insulin, Maxolon should be discontinued.
Young adults aged 15-19 years.
The use of Maxolon in young adults 15-19 years should be restricted to the following situations and only used as second line therapy.
Severe intractable vomiting of known cause.
Vomiting associated with radiotherapy and intolerance to cytotoxic drugs.
As an aid to gastrointestinal intubation.4.2 Dose and Method of Administration
Tablets should not be used in children less than 15 years.
Patients with normal renal and hepatic function.
The dosage recommendations given below should be strictly adhered to if side effects of the dystonic type are to be avoided. Total daily dosage of Maxolon, especially for young adults should not normally exceed 0.5 mg/kg bodyweight with a maximum of 30 mg daily. Maxolon should only be used after careful examination to avoid masking an underlying disorder, e.g. cerebral irritation. Maximum recommended treatment duration is 5 days.
Medical indications.
Adults 20 years and over.
Maximum of 10 mg three times daily.
Elderly patients.
As for adults. To avoid adverse reactions adhere strictly to dosage recommendations and where prolonged therapy is considered necessary, patients should be regularly reviewed.
Young adults 15-19 years.
5 to 10 mg three times daily, commencing at the lower dosage and used as second line therapy only.
Diagnostic indications.
A single dose of Maxolon may be given 5 to 10 minutes before the examination. Subject to bodyweight considerations, the following dosages are recommended.
Adults 20 years and over.
10 to 20 mg.
Young adults 15-19 years.
10 mg.
Patients with impaired renal and hepatic function.
In patients with clinically significant degrees of renal or hepatic impairment, clearance of Maxolon is likely to be reduced. It is suggested that therapy be initiated at half the recommended dose. Subsequent dosage will depend on individual clinical response.4.3 Contraindications
Maxolon should not be used whenever stimulation of gastrointestinal motility might be dangerous, e.g. in the presence of gastrointestinal haemorrhage, mechanical obstruction, or perforation.
Phaeochromocytoma because Maxolon may cause a hypertensive crisis, probably due to release of catecholamines from the tumour. Such hypertensive crises may be controlled by phentolamine.
Known hypersensitivity or intolerance to the drug.
Porphyria.
Epilepsy as Maxolon may increase the frequency and severity of seizures.
Maxolon should not be administered to patients receiving other drugs which are likely to cause extrapyramidal reactions, since the frequency and severity of extrapyramidal reactions may be increased.
Maxolon tablets should not be used in children below 15 years of age.
4.4 Special Warnings and Precautions for Use
Persistent tardive dyskinesia.
Tardive dyskinesia may appear in some patients on long-term therapy or may appear after drug therapy has been discontinued. The risk appears to be greater in elderly patients on high dose therapy, especially females. The symptoms are persistent and can oftentimes appear to be irreversible. The syndrome is characterised by rhythmical involuntary movement of the tongue, face, mouth or jaw (e.g. protrusion of tongue, puffing of cheeks, puckering of mouth, chewing movements). Sometimes these may be accompanied by involuntary movement of extremities. There is no known effective treatment for tardive dyskinesia, however, in some patients symptoms may lessen or resolve after Maxolon treatment is stopped. Antiparkinson agents usually do not alleviate the symptoms of this syndrome.
Although the risk of tardive dyskinesia with metoclopramide has not been extensively studied, one published study reported a tardive dyskinesia prevalence of 20% among patients treated for at least 3 months. Both the risk of developing the syndrome and the likelihood that it will become irreversible are believed to increase with the duration of treatment and the total cumulative dose.
Maxolon therapy should routinely be discontinued in patients who develop signs or symptoms of tardive dyskinesia. It has been suggested that fine vermicular movements of the tongue may be an early sign of the syndrome, and, if the medication is stopped at that time, the syndrome may not develop. Tardive dyskinesia may remit, partially or completely, within several weeks to months after metoclopramide is withdrawn. Metoclopramide itself, however, may suppress (or partially suppress) the signs of tardive dyskinesia, thereby masking the underlying disease process. The effect of this symptomatic suppression upon the long-term course of the syndrome is unknown. Therefore, metoclopramide should not be used for the symptomatic control of tardive dyskinesia.
Prolonged treatment (greater than 12 weeks) with metoclopramide should be avoided in all but rare cases where therapeutic benefit is thought to outweigh the risks to the patient of developing tardive dyskinesia.
Care should be exercised in patients being treated with other centrally active drugs.
Since extrapyramidal symptoms may occur with both Maxolon and neuroleptics such as phenothiazines, care should be exercised in the event of both drugs being prescribed concurrently (see Section 4.5 Interactions with Other Medicines and Other Forms of Interactions). The frequency and severity of seizures or extrapyramidal reactions may be increased in epileptic patients given Maxolon.
Dystonic reactions.
Dystonic reactions occur in approximately 1% of patients given Maxolon. These occur more frequently in children and young adults and may occur after a single dose.
Neuroleptic malignant syndrome (NMS).
NMS has been reported with Maxolon in combination with neuroleptics as well as with Maxolon monotherapy (see Section 4.8 Adverse Effects (Undesirable Effects)).
Prolactin levels.
Maxolon elevates prolactin levels and the elevation persists during chronic administration (see Section 4.8 Adverse Effects (Undesirable Effects)). Tissue culture experiments indicate that approximately one-third of human breast cancers are prolactin dependent in vitro, a factor of potential importance if the prescription of Maxolon is contemplated in a patient with previously detected breast cancer. Although disturbances such as galactorrhoea, amenorrhoea, gynaecomastia, and impotence have been reported with prolactin elevating drugs, the clinical significance of elevated serum prolactin levels is unknown for most patients. An increase in mammary neoplasms has been found in rodents after chronic administration of prolactin stimulating neuroleptic drugs. Neither clinical studies nor epidemiological studies conducted to date, however, have shown an association between chronic administration of these drugs and mammary tumorigenesis; the available evidence is too limited to be conclusive at this time.
Other.
Following operations such as pyloroplasty or gut anastomosis, Maxolon therapy should be withheld for three or four days as vigorous muscular contractions may not help healing.
The symptomatic relief provided by Maxolon may delay recognition of serious disease. It should not be prescribed until diagnosis has been established and should not be substituted for appropriate investigation of the patient's symptoms.
If vomiting persists in a patient receiving Maxolon, the patient should be reassessed to exclude the possibility of an underlying disorder e.g. cerebral irritation.
Metoclopramide induced depression has been reported in patients without a prior history of depression. Metoclopramide should be given to patients with a prior history of depression only if the expected benefits outweigh the potential risks.
Metoclopramide should be used with caution in patients with hypertension as intravenously administered metoclopramide has been shown to release catecholamines.
Metoclopramide can exacerbate parkinsonian symptoms, therefore it should be used with caution, if at all, in patients with parkinsonian syndrome (see Section 4.8 Adverse Effects (Undesirable Effects)).
Use in hepatic impairment.
In patients with clinically significant degrees of hepatic impairment, clearance of Maxolon is likely to be reduced (see Section 4.2 Dose and Method of Administration).
Use in renal impairment.
In patients with clinically significant degrees of renal impairment, clearance of Maxolon is likely to be reduced.
Special care should be taken in cases of severe renal insufficiency (see Section 4.2 Dose and Method of Administration).
Use in the elderly.
To avoid adverse reactions adhere strictly to dosage recommendations and where prolonged therapy is considered necessary, patients should be regularly reviewed (see Section 4.2 Dose and Method of Administration).
Paediatric use.
Maxolon tablets are contraindicated in children less than 15 years of age because of the higher incidence of adverse reactions in this age group.
Effects on laboratory tests.
No data available.4.5 Interactions with Other Medicines and Other Forms of Interactions
The effects of Maxolon on gastrointestinal motility are antagonised by anticholinergic drugs and narcotic analgesics. Additive sedative effects can occur when Maxolon is given with alcohol, sedatives, hypnotics, narcotics or tranquillisers.
Since Maxolon accelerates abnormally slow gastric and small bowel peristaltic activity, it may change absorption of orally administered drugs. The absorption of drugs from the small bowel may be accelerated (e.g. paracetamol, tetracycline, L-dopa), whereas absorption of drugs from the stomach may be diminished (e.g. digoxin).
Metoclopramide may cause extrapyramidal symptoms in some patients. Therefore, when metoclopramide is used concomitantly with other drugs that are likely to cause extrapyramidal reactions (e.g. neuroleptics such as phenothiazines), caution should be exercised.
The decrease in gastric emptying time caused by metoclopramide may increase the bioavailability of ciclosporin. Monitoring of ciclosporin concentrations may be necessary.
When metoclopramide is given concurrently with suxamethonium the recovery time is prolonged.
Since metoclopramide influences the delivery of food to the intestine and thus, the rate of its absorption, the administration of metoclopramide may result in poor diabetic control in some patients. Therefore adjustment in, or timing of, insulin dosage may be necessary in insulin controlled diabetics.
The finding that metoclopramide releases catecholamines in patients with essential hypertension suggests that it should be used cautiously, if at all, in patients receiving monoamine oxidase inhibitors.
4.6 Fertility, Pregnancy and Lactation
Effects on fertility.
No data available.
(Category A)
Adequate human data on use during pregnancy are not available.
Adequate human data on use during lactation and adequate animal reproduction studies are not available.4.7 Effects on Ability to Drive and Use Machines
Patients should be cautioned about engaging in activities requiring mental alertness for a few hours after the drug has been administered.
4.8 Adverse Effects (Undesirable Effects)
The most frequent adverse reactions to Maxolon are restlessness, drowsiness, fatigue and lassitude, which occur in approximately 10% of patients.
Less frequently, insomnia, headache, dizziness, nausea, or bowel disturbances may occur. Rare (less than 1 in 1,000) cases of acute depression have been reported. Anxiety or agitation may occur, especially after rapid injection.
A single instance of supraventricular tachycardia following intramuscular administration has been reported. There have been very rare (less than 1 in 10,000) cases of abnormalities of cardiac conduction (such as bradycardia and heart block) in association with intravenous metoclopramide. Atrial fibrillation (AF), oedema, tachycardia and palpitations have been associated with the use of metoclopramide.
Although uncommon at normal dosage, various extrapyramidal reactions to Maxolon, usually of the dystonic type, have been reported. Reactions include: spasm of the facial muscles, trismus, rhythmic protrusion of the tongue, a bulbar type of speech, spasm of the extraocular muscles including oculogyric crises, unnatural positioning of the head and shoulders and opisthotonos. There may be a generalised increase in muscle tone. The majority of reactions occur within 36 hours of starting treatment and the effects usually disappear within 24 hours of withdrawal of the drug, however, close observation is required and in cases of more severe reactions, an antiparkinson drug such as benztropine or an anticholinergic antihistamine such as diphenhydramine should be given. A fatal dystonic reaction has been reported in a patient who received hexamethylmelamine, cisplatin and high dose metoclopramide. A fatal cardiorespiratory arrest has occurred in at least one patient with an acute dystonic reaction.
Tardive dyskinesia, which may be persistent, has been reported, particularly in elderly patients undergoing long-term therapy with Maxolon.
Very rare (less than 1 in 10,000) occurrences of the Neuroleptic Malignant Syndrome have been reported. This syndrome is potentially fatal and comprises hyperpyrexia, altered consciousness, muscle rigidity, autonomic instability and elevated levels of CPK and must be treated urgently (recognised treatments include dantrolene and bromocriptine). Maxolon should be stopped immediately if this syndrome occurs.
Parkinsonian symptoms, including tremor, rigidity, bradykinesia and akinesia, occur rarely in patients receiving metoclopramide but may be associated with usual or excessive doses or with decreased renal function.
There have been isolated reports of hypersensitivity reactions (such as urticaria, maculopapular rash) in patients receiving metoclopramide.
Methaemoglobinaemia has also been reported. There have been a few cases of neutropenia, leucopenia and agranulocytosis generally without clear cut relationship to metoclopramide.
Sulfhaemoglobinaemia in adults.
Hyperthermia has also been observed.
Raised serum prolactin levels have been observed during metoclopramide therapy; this effect is similar to that noted with many other compounds. Galactorrhoea and breast enlargement have also been observed during metoclopramide therapy.
Respiratory failure, secondary to dystonic reaction, acute asthmatic symptoms of wheezing and dyspnoea may occur.
Urinary incontinence and frequency, sexual dysfunction, priapism and muscle spasm may also occur.
Rarely, cases of hepatotoxicity, characterised by such findings as jaundice and altered liver function tests, when metoclopramide was administered with other drugs with known hepatotoxic potential.
Reporting suspected adverse effects.
Reporting suspected adverse reactions after registration of the medicinal product is important. It allows continued monitoring of the benefit-risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions at www.tga.gov.au/reporting-problems.4.9 Overdose
For information on the management of overdose contact the Poisons Information Centre on 131126 (Australia).
Symptoms.
Overdose of metoclopramide may be expected to produce effects that are extensions of common adverse reactions: drowsiness, disorientation and extrapyramidal side effects. Very rarely AV block has been observed. Other reported effects associated with metoclopramide overdose have included feelings of anxiety or restlessness, headache, vertigo, nausea, vomiting, constipation, weakness, hypotension and xerostomia.
Management.
Management of overdosage consists of close observation and supportive therapy. Antiparkinson and antihistamine/anticholinergic drugs such as diphenhydramine hydrochloride have effectively controlled extrapyramidal reactions. Haemodialysis appears ineffective in removing metoclopramide. Similarly, continuous ambulatory peritoneal dialysis does not remove significant amounts of the drug.5 Pharmacological Properties
5.1 Pharmacodynamic Properties
Mechanism of action.
Maxolon stimulates motility of the upper gastrointestinal tract without stimulating gastric, biliary or pancreatic secretions. Its mode of action is unclear. It seems to sensitise tissues to the action of acetylcholine. The effect of Maxolon on motility is not dependent on intact vagal innervation, but it can be abolished by anticholinergic drugs.
Maxolon increases the tone and amplitude of gastric (especially antral) contractions, relaxes the pyloric sphincter and the duodenal bulb, and increases peristalsis of the duodenum and jejunum resulting in accelerated gastric emptying and intestinal transit. It increases the resting tone of the lower oesophageal sphincter. It has little, if any effect on the motility of the colon or gall bladder.
Maxolon has dopamine antagonist activity. Like the phenothiazines and related drugs, which are also dopamine antagonists, Maxolon produces sedation and may produce extra-pyramidal reactions (see Section 4.4 Special Warnings and Precautions for Use). Maxolon inhibits the central and peripheral effects of apomorphine, induces release of prolactin and causes a transient increase in circulating aldosterone levels.
Clinical trials.
No data available.
5.2 Pharmacokinetic Properties
Absorption.
The onset of pharmacological action is 30 to 60 minutes following an oral dose; pharmacological effects persist for 1 to 2 hours. There is marked variability in peak plasma concentrations of Maxolon after oral administration, which appears to be due to interindividual differences in first-pass metabolism.
Distribution.
Plasma protein binding is 13 to 22%.
Metabolism.
About 80% of the drug is excreted in the urine in the first 24 hours, approximately half as the glucuronide and sulfate conjugates and half as unchanged drug.
Excretion.
Elimination half-life varies in different studies from 2.5 to 5 hours. Impaired renal function results in reduced clearance of Maxolon and an increased half-life (15 hours).
5.3 Preclinical Safety Data
Genotoxicity.
No data available.
Carcinogenicity.
No data available.6 Pharmaceutical Particulars
6.1 List of Excipients
Colloidal anhydrous silica, lactose monohydrate, magnesium stearate, maize starch, pregelatinised maize starch.
6.2 Incompatibilities
Incompatibilities were either not assessed or not identified as part of the registration of this medicine.
6.3 Shelf Life
In Australia, information on the shelf life can be found on the public summary of the Australian Register of Therapeutic Goods (ARTG). The expiry date can be found on the packaging.
6.4 Special Precautions for Storage
Store below 30°C.
6.5 Nature and Contents of Container
PVC/Al Blister pack 25's, 100's.
6.6 Special Precautions for Disposal
In Australia, any unused medicine or waste material should be disposed of by taking to your local pharmacy.
6.7 Physicochemical Properties
Chemical structure.
 C14H22ClN3O2, HCl, H2O.
C14H22ClN3O2, HCl, H2O.
Chemical name: N-(diethyl-aminoethyl)-2-methoxy-4-amino-5- chlorbenzamide monohydrochloride monohydrate.
CAS number.
54143-57-6.7 Medicine Schedule (Poisons Standard)
S4 Prescription Only Medicine.
Summary Table of Changes


 C14H22ClN3O2, HCl, H2O.
C14H22ClN3O2, HCl, H2O.