What is in this leaflet
This leaflet answers some common questions about Mersyndol.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor or pharmacist has weighed the risks of you taking this medicine against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What Mersyndol is used for
Mersyndol is a type of analgesic intended for short term use to relieve moderate pain and fever.
Paracetamol and codeine work together to stop the pain messages from getting through to the brain. Doxylamine is an antihistamine with calmative effects.
Your doctor, however, may prescribe Mersyndol for another purpose.
Ask your doctor or pharmacist if you have any questions about why it has been prescribed for you.
This medicine may be habit-forming if taken frequently or over long periods.
Before you take it
When you must not take it
Do not take Mersyndol if you have:
- an allergic reaction to paracetamol, codeine or doxylamine
- respiratory depression (shallow breathing) or respiratory insufficiency (difficulty breathing)
- severe liver failure or impaired liver function
- G6PD deficiency, a human enzyme deficiency
- known CYP 2D6 ultra-rapid metaboliser (a fast metaboliser of codeine by the CYP 2D6 enzyme)
- are aged between 12-18 years of age and may have lowered respiratory function including having had your tonsils or adenoids removed
- using antidepressant medication (Monoamine inhibitors (MAOIs)), or have stopped taking antidepressant medication within the past 14 days
Do not take Mersyndol if you are allergic to it or any of the ingredients listed at the end of this leaflet. Some symptoms of an allergic reaction include skin rash, itching, shortness of breath or swelling of the face, lips or tongue, which may cause difficulty in swallowing or breathing.
Do not give Mersyndol to children under 12 years of age.
Do not take Mersyndol during the third trimester of pregnancy.
Do not take Mersyndol during labour, especially if the baby is premature. This medicine may produce withdrawal effects in the newborn baby.
Do not take it if you are breastfeeding or planning to breastfeed. Mersyndol passes into breast milk and there is a possibility your baby may be affected.
Do not take it after the expiry date (EXP) printed on the pack. If you take it after the expiry date has passed, it may not work as well.
Do not take it if the packaging is torn/damaged or shows signs of tampering.
Before you start to take it
Tell your doctor or pharmacist if you have allergies to:
- any of the ingredients listed at the end of this leaflet
- any other medicines
- aspirin or any other NSAID medicine
- any other substances, such as foods, preservatives or dyes.
Tell your doctor or pharmacist if you are pregnant or intend to become pregnant. Like most medicines of this kind, Mersyndol is not recommended to be used during pregnancy. Your doctor or pharmacist will discuss the risks and benefits of taking it if you are pregnant.
Tell your doctor or pharmacist if you have or have had any medical conditions, especially the following:
- liver problems
- kidney problems
- heart problems
- low blood pressure
- difficulty breathing, wheezing, chronic cough, asthma, or other chronic breathing conditions
- compromised respiratory function (due to emphysema, kyphoscoliosis or obesity)
- known analgesic intolerance
- you are a CYP 2D6 ultra-rapid metaboliser
- chronic alcohol use including recent cessation of alcohol intake
- low glutathione reserves
- Gilbert's syndrome
- prostate problems
- thyroid problems
- Multiple sclerosis
- urinary, bowel or gallbladder conditions
- have problems with the adrenal glands
- convulsions, fits or seizures
- pre-existing opioid dependence
- chronic constipation
- head injury or trauma
- a history of drug dependence, including alcohol dependence. Caution is particularly recommended for use in adolescents and young adults with a history of drug and/or alcohol abuse.
- prone to angle closure glaucoma (high pressure in the eye)
- difficulty or inability to pass urine
Tell your doctor or pharmacist if you plan to have surgery.
If you have not told your doctor or pharmacist about any of the above, tell them before you take Mersyndol.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you buy without a prescription from your pharmacy, supermarket or health food store.
Some medicines may interfere with the absorption of Mersyndol.
These include:
- Antihistamines
- Sleeping tablets
- Tranquillisers (medicines used for anxiety or nerves)
- Benzodiazepines (medicines used as sedatives or to treat anxiety)
- Medicines containing alcohol (ethanol), e.g. some cough syrups
- Any medicine which thins the blood
- other opioid analgesics used to treat pain
- monoamine oxidase inhibitors, medicine used to treat depression, taken within the last 14 days
- Antihypertensives (medicines used to help lower blood pressure)
- Medicines to treat epilepsy
- Metoclopramide or domperidone, medicines used to control nausea and vomiting
- Propantheline, a medicine used to treat stomach ulcers
- Chloramphenicol (antibiotic used to treat ear and eye infections)
- Flucloxacillin, zidovudine or rifampicin, medicines used to treat infections
- Antidepressants
- Antipsychotics (medicines used to treat mental illnesses)
- Chelating resin
- medicines used to treat alcohol and/or opioid dependence (e.g. naltrexone, buprenorphine or methadone)
- CYP 2D6 inhibitors such as quinidine, fluoxetine, paroxetine, bupropion, cinacalcet, methadone
- CYP 3A4 inducers such as rifampin
These medicines may be affected by Mersyndol, or may affect how well it works. You may need to use different amounts of your medicine, or take different medicines. Your doctor or pharmacist will advise you.
Your doctor or pharmacist has more information on medicines to be careful with or to avoid while taking Mersyndol.
How to take it
How much to take
The standard dose of this medicine for adults and children 12 years or over is one or two tablets/caplets every 4 to 6 hours, as needed for pain relief.
Do not take more than 8 tablets/caplets in a 24 hour period.
Mersyndol is not recommended to be used for long periods of time.
Your doctor may have prescribed a different dose.
Ask your doctor or pharmacist if you are unsure of the correct dose for you. They will tell you exactly how many to take.
Follow the instructions they give you. If you take the wrong dose, Mersyndol may not work as well and your problem may not improve.
Mersyndol is not recommended for use in children under 12 years of age.
How to take it
Swallow the tablets whole with a full glass of water or other liquid.
When to take it
Mersyndol can be taken with or without food.
If you are not sure when to take it, ask your doctor or pharmacist.
If you forget to take it
Do not try to make up for missed doses by taking more than one dose at a time. This may increase the chance of getting an unwanted side effect.
If it is almost time for your next dose, skip the dose you missed and take the next dose when you are meant to.
Do not take a double dose to make up for the dose you have missed.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering when to take your medicine, ask your pharmacist for hints.
If you take too much (overdose)
Immediately telephone your doctor, or the Poisons Information Centre (telephone Australia 13 11 26 or New Zealand 0800 POISON or 0800 764 766), or go to Accident and Emergency at your nearest hospital, if you think you or anyone else may have taken too much Mersyndol.
Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention. Large amounts of paracetamol can cause liver damage.
If children take too many Mersyndol they can suffer from nightmares, hallucinations, fitting or have difficulty sleeping.
While you are taking it
Things you must do
Tell all the doctors, dentists and pharmacists who are treating you that you are taking Mersyndol.
If you are about to be started on any new medicine, tell your doctor and pharmacist that you are taking Mersyndol.
If you plan to have surgery that needs a general anaesthetic, tell your doctor or dentist that you are taking this medicine.
If you become pregnant while you are taking this medicine, tell your doctor or pharmacist that you are taking Mersyndol.
Things you must not do
Do not take more than the recommended dose unless your doctor or pharmacist tells you to.
Do not give this medicine to anyone else, even if they have the same condition as you.
Do not use this medicine to treat any other complaints unless your doctor or pharmacist tells you to.
Things to be careful of
Mersyndol may cause dizziness, drowsiness or light-headedness in some people, especially after the first dose. Do not drive a car, operate machinery, or do anything else that could be dangerous if you feel dizzy.
Children should not ride bicycles if affected and should be supervised to avoid potential harm.
Be careful if you are over 65 and unwell or taking other medicines. Some people may experience side effects such as drowsiness, confusion, dizziness and unsteadiness, which may increase the risk of a fall.
Drinking alcohol increases the likelihood of becoming drowsy while taking Mersyndol. Drinking alcohol and taking paracetamol at the same time can cause liver damage. It is not recommended that you drink alcohol while taking Mersyndol.
Mersyndol may be habit forming if taken at high doses for extended periods of time. Ask your doctor or pharmacist if you are concerned about this.
Side effects
All medicines have some unwanted side effects. Sometimes they are serious, but most of the time they are not. Your doctor or pharmacist has weighed the risks of using this medicine against the benefits they expect it will have for you.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Mersyndol.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- Drowsiness
- Dizziness
- Nausea
- Vomiting
- Stomach pain
- Constipation
- Skin rashes
- Sweating
- Diarrhoea
- Dry mouth
- Indigestion
- Ringing in the ear
- Headache
- Depression
- Increased sensitivity to pain or increased levels of pain
- Blurred vision
- Visible slowing of physical and emotional reactions
- Thickened phlegm
- Difficulty or inability to pass urine
Tell your doctor as soon as possible if you notice any of the following:
- Painful red areas with blisters and peeling layers of skin which may be accompanied by fever and/or chills
- Severe blisters and bleeding in the lips, eyes, mouth, nose and genitals
- Hepatitis (symptoms include loss of appetite, itching, yellowing of the skin and eyes, light coloured bowel motions, dark coloured urine)
- Difficulty breathing
- Flushing of the face
- Unusual or extreme mood swings
- Feeling confused
- Dizziness, light-headedness
- Fast heartbeat
If any of the following happen, stop taking this medicine and tell your doctor immediately, or go to Accident and Emergency at your nearest hospital:
- swelling of the face, lips, mouth or throat, which may cause difficultly in swallowing or breathing
- hives
- fainting
- yellowing of the skin and eyes (jaundice)
These are very serious side effects. If you have them, you may have had a serious allergic reaction to Mersyndol. You may need urgent medical attention or hospitalisation.
These side effects are very rare.
Tell your doctor or pharmacist if you notice anything else that is making you feel unwell. Other side effects not listed above may occur in some consumers.
Ask your doctor or pharmacist to answer any questions you may have.
After taking it
If you have any queries about any aspect of your medicine, or any questions regarding the information in this leaflet, discuss them with your doctor or pharmacist.
Storage
Keep your tablets/caplets in the blister pack until it is time to take them. If you take the tablets/caplets out of the box or the blister pack they may not keep well.
Keep the medicine in a cool, dry place where the temperature stays below 30°C.
Do not store it or any other medicine in the bathroom, near a sink, or on a windowsill.
Do not leave it in the car. Heat and damp can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor or pharmacist tells you to stop taking Mersyndol, or the medicine has passed its expiry date, ask your pharmacist what to do with any that are left over.
Product description
What it looks like
Mersyndol is available as tablets or caplets.
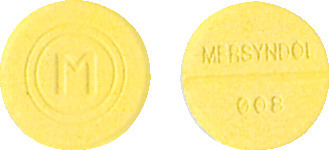
- Tablets - yellow, flat and round with 'M' inside two circles on one side and 'Mersyndol 008' and a breakline on the other side
- Caplets - yellow, capsule-shaped tablets with 'Mersyndol' on one side and a breakline on the other side
Tablets and caplets are available in a box containing 20 or 40 tablets/caplets.
Ingredients
Active Ingredient:
Each Mersyndol tablet and caplet contains:
- Paracetamol 450 mg
- Codeine phosphate hemihydrate 9.75 mg
- Doxylamine succinate 5 mg
Inactive Ingredients:
Each Mersyndol tablet and caplet contains:
- microcrystalline cellulose
- purified talc
- magnesium stearate
- sodium starch glycollate
- colouring agents - quinoline yellow and sunset yellow FCF
- COMPAP L (PI 910)
Mersyndol does not contain aspirin, gluten, sucrose, lactose, tartrazine or any other azo dyes.
Manufacturer/Sponsor
Mersyndol is supplied in Australia by:
sanofi-aventis australia pty ltd
12-24 Talavera Road
Macquarie Park NSW 2113
Mersyndol is supplied in New Zealand by:
sanofi-aventis new zealand limited
Level 8,
56 Cawley Street
Ellerslie
Auckland
This leaflet was prepared in July 2020.
Australian Register Numbers
Tablets: AUST R 10110
Caplets: AUST R 56535
® Registered Trademark
mersyndol-ccdsv4-ccsiv1-cmiv13-03jul20
Published by MIMS October 2020