What is in this leaflet
This leaflet answers some common questions about this medicine. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking this medicine against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What this medicine is used for
The name of your medicine is METOPROLOL-WGR tablets. It contains the active ingredient metoprolol.
It is used:
- to treat high blood pressure, also called hypertension
- to prevent a type of chest pain, also called angina
- after a heart attack
- to prevent migraine headaches.
Metoprolol belongs to a group of medicines called beta-blockers. It works by affecting the body's response to some nerve impulses, especially in the heart.
As a result, it decreases the heart's need for blood and oxygen and reduces the amount of work that the heart must do. It also widens the blood vessels in the rest of the body.
Metoprolol can be used alone or in combination with other medicines to treat your condition.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
Metoprolol is only available with a doctor's prescription.
There is no evidence that this medicine is addictive.
Use in children
Metoprolol is not recommended for use in children.
Before you take this medicine
When you must not take it
Do not take this medicine if you have or have had any of the following medical conditions:
- sudden losses of consciousness
- asthma, wheezing, difficulty breathing or other severe lung problems
- a history of allergic problems, including hayfever
- a very slow heartbeat (less than 45 to 50 beats per minute)
- low blood pressure
- a severe blood vessel disorder causing poor circulation in the arms and legs, severe drops in blood pressure, dizziness, fast heartbeat, rapid and shallow breathing or cold clammy skin
- phaeochromocytoma (a rare tumour of the adrenal gland) which is not already being treated with other medicines
- sudden and oppressive chest pain, a sign of heart attack
- irregular heartbeat (without functioning pacemaker)
- other heart disorders
- swollen ankles and/or tiredness due to heart disease or certain other heart conditions
- poor blood circulation in your limbs (e.g. very cold, pale hands or feet, or pain in your leg muscles when you walk)
- shock
If you are not sure whether any of the above medical conditions apply to you, check with your doctor.
Do not take this medicine if you have an allergy to:
- any medicine containing metoprolol
- any other beta-blocker medicines
- any of the ingredients listed at the end of this leaflet
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin
Do not take this medicine if you are pregnant.
Metoprolol should not be used throughout pregnancy, especially during the first 3 months of pregnancy, unless clearly necessary.
Metoprolol may affect your baby, especially if you take it in the last few days before your baby is born.
Your doctor can discuss with you the risks and benefits involved.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives, dyes or bee or wasp stings.
Your doctor will want to know if you are prone to allergies. Beta-blocker medicines can make an allergic reaction worse.
Tell your doctor if you have or have had any of the following medical conditions:
- diabetes
- an overactive thyroid gland
- kidney problems
- liver problems
- a heart attack
- chest pain when you are at rest, or certain types of angina, such as Prinzmetal angina or variant angina
- oculomucocutaneous syndrome (signs include severe conjunctivitis and skin rash and ear infection)
Tell your doctor if you are pregnant or plan to become pregnant or are breastfeeding.
Metoprolol passes into breast milk and there is a possibility that your baby could be affected.
Your doctor can discuss with you the risks and benefits involved.
If you have not told your doctor about any of the above, tell them before you start taking this medicine.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interact with metoprolol. These include:
- other beta-blocker medicines, including beta-blocker eye drops
- other medicines used to treat high blood pressure e.g. calcium channel blockers (such as verapamil or diltiazem), clonidine and hydralazine
- some medicines used to treat angina
- adrenaline or similar substances, which are often found in eye or nose drops, or in some cough and cold medicines
- other medicines used to treat irregular heart beat (arrhythmias), e.g. amiodarone, flecainide, disopyramide and quinidine
- medicines for diabetes
- quanethidine, used to treat certain heart conditions
- some local and general anaesthetics used during surgery
- warfarin or dipyridamole, used to prevent blood clots
- non-steroidal anti-inflammatory drugs (NSAIDs), used to relieve pain or inflammation e.g. ibuprofen or indomethacin
- cimetidine, used for stomach ulcers
- some antibiotics (e.g. rifampicin)
- some antivirals (e.g. ritonavir)
- some antihistamines (e.g. diphenhydramine)
- some antidepressant medications (e.g. fluoxetine, paroxetine or bupropion)
- monoamine-oxidase inhibitor (MAOI) medicines
- some antifungals (e.g. terbinafine)
- ergot alkaloids, used in the prevention and treatment of migraine headaches
These medicines may be affected by metoprolol or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
How to take this medicine
Follow carefully all directions given to you by your doctor. Their instructions may be different to the information in this leaflet.
How much to take
Your doctor will tell you how much of this medicine you should take. This will depend on your condition and whether you are taking any other medicines.
High blood pressure:
The usual dose is from 50 mg to 200 mg each day, either as a single dose or divided into two doses.
Angina:
The usual dose is from 100 mg to 300 mg each day, divided into two or three doses.
After a heart attack:
The usual dose is 200 mg each day, divided into two doses.
To prevent migraine:
The usual dose is from 100 mg to 150 mg each day, divided into two doses (morning and evening).
How to take it
Swallow the tablets whole with a full glass of water.
It does not matter if you take metoprolol before or after food.
How long to take it
Continue taking metoprolol for as long as your doctor tells you.
Metoprolol helps to control your symptoms, but it does not cure your condition. Your doctor will check your progress to make sure the medicine is working and will decide how long your treatment should continue.
Talk to your doctor if you are not sure how long you need to take your medicine for.
If you forget to take it
If it is almost time for your next dose, skip the missed dose and take your next dose at the usual time.
Otherwise, take it as soon as you remember, and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for the one that you missed. This may increase the chance of you getting an unwanted side effect.
If you have trouble remembering when to take your medicine, ask your pharmacist for some hints to help you remember.
If you take too much
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much of this medicine. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
Symptoms of an overdose may include feeling sick and vomiting, bluish skin and nails, very low blood pressure, slow heartbeat, difficulty breathing, fainting, convulsions (fits), coma or death.
While you are taking this medicine
Things you must do
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking this medicine.
Tell any other doctors, dentists, and pharmacists who treat you that you are taking this medicine.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking this medicine. It may affect other medicines used during surgery.
If you become pregnant or start to breastfeed while taking this medicine, tell your doctor immediately.
If you have an allergic reaction to a food, another medicine or an insect sting while you are taking this medicine, tell your doctor immediately. There is a chance that metoprolol could make the allergic reaction worse or harder to treat.
If you feel light-headed, dizzy or faint when getting out of bed or standing up, get up slowly.
You may feel light-headed or dizzy when you start to take metoprolol.
This is because your blood pressure is falling suddenly. If this problem doesn't go away, talk to your doctor.
To avoid symptoms of low blood pressure, here are some hints that may help:
- Stand up slowly to help your body get used to the change in position and blood pressure
- If you feel dizzy, sit or lie down until you feel better
- If you feel faint, breathe deeply and bend forward with your head between your knees
- Take extra care when exercising, driving or standing for long periods, especially in hot weather. Drink plenty of fluids, especially if you sweat a lot.
If you are being treated for diabetes, make sure you check your blood sugar regularly and report any problems to your doctor. Metoprolol may change how well your diabetes is controlled. It may also prevent some of the warning signs of low blood sugar, such as fast heartbeat and may make low blood sugar last longer. The dose of your diabetes medicines may need to be changed.
If you are about to have any blood tests, tell your doctor that you are taking this medicine. It may interfere with the results of some tests.
Make sure you drink enough water during exercise and hot weather when you are taking this medicine, especially if you sweat a lot. If you do not drink enough water while taking metoprolol, you may feel faint or lightheaded or sick. This is because your blood pressure is dropping too much. If you continue to feel unwell, tell your doctor.
Keep all your doctor's appointments so that your progress can be checked. Your doctor may do some tests from time to time to make sure the medicine is working and to prevent unwanted side effects.
Things you must not do
Do not take this medicine to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or lower the dosage without checking with your doctor. Your doctor may want to gradually reduce the amount of metoprolol you are taking before stopping it completely. This helps to reduce the chance of your condition becoming worse or keep other unwanted heart problems from happening.
Things to be careful of
Be careful driving, operating machinery or doing jobs that require you to be alert while you are taking this medicine until you know how it affects you.
As with other beta-blocker medicines, metoprolol may cause dizziness, light-headedness or decreased alertness in some people.
If you have any of these symptoms, do not drive or do anything else that could be dangerous.
Be careful drinking alcohol while you are taking this medicine. If you drink alcohol, dizziness or light-headedness may be worse.
Be careful to dress warmly during cold weather, especially if you will be outside for a long time. Like other beta-blocker medicines, metoprolol may make you more sensitive to cold temperatures, especially if you have problems with your blood circulation. These medicines tend to decrease blood circulation in the skin, fingers and toes.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking this medicine.
All medicines can have side effects. Sometimes they are serious but most of the time they are not.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
If you are over 65 years of age, you may have an increased chance of getting side effects.
Tell your doctor if you notice any of the following and they worry you:
- tiredness, drowsiness or decreased alertness
- dizziness, spinning sensation (vertigo) or light-headedness
- headache or other aches and pains
- sleepiness during the day, difficulty sleeping or nightmares
- stomach ache or upset, nausea (feeling sick) or vomiting
- diarrhoea or constipation
- dry or irritated eyes; blurred vision
- dry mouth
- increased sweating
- runny or blocked nose
- abnormal triglycerides, cholesterol values or liver function tests during treatment with metoprolol
Tell your doctor as soon as possible if you notice any of the following:
- fainting
- depression or other changes in mood
- confusion or loss of memory
- buzzing or ringing in the ears, or other difficulty hearing
- problems with sexual function
- weight gain
- hair thinning
- worsening of psoriasis
- muscle cramps or painful joints
- a tingling sensation
The above list includes serious side effects that may require medical attention.
Tell your doctor immediately or go to Accident and Emergency at your nearest hospital if you notice any of the following:
- shortness of breath, sometimes with tiredness, weakness or reduced ability to exercise
- swelling of the feet or legs due to fluid build-up
- coldness, burning, numbness or pain in arms and legs
- chest pain
- pain behind the breastbone (different from angina)
- changes in heart rate (fast, slow or irregular)
- yellowing of the skin or eyes (jaundice), sometimes with pain in the abdomen
- constant "flu-like" symptoms (chills, fever, sore throat, aching joints, swollen glands, tiredness or lack of energy)
- unusual bleeding or bruising
- skin reactions (rash, itching or worsening of psoriasis)
- symptoms of sunburn (redness, itching, swelling or blistering) that happen much more quickly than normal
- abnormal thinking or hallucinations (seeing or hearing things that are not there)
- symptoms of an allergic reaction including cough, shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue, throat or other parts of the body; rash, itching or hives on the skin
Other side effects not listed above may occur in some patients.
Storage and Disposal
Storage
Keep your tablets in the original pack, away from direct light, until it is time to take them. If you take your medicine out of its original packaging it may not keep well.
Keep your medicine in a cool dry place where the temperature will stay below 30°C.
Do not store your medicine, or any other medicine, in the bathroom or near a sink. Do not leave it in the car or on windowsills. Heat and dampness can destroy some medicines.
Keep this medicine where children cannot reach it. A locked cupboard at least one and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What METOPROLOL-WGR looks like
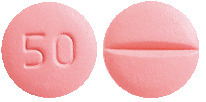
Metoprolol 50 mg tablets:
Pink, round, biconvex film-coated tablets with notch break line on one side and '50' debossed on the other side. AUST R 234003.
A blister pack contains 10 tablets or 100 tablets.
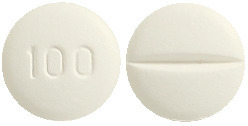
Metoprolol 100 mg tablets:
White to off white, round, biconvex film-coated tablets with notch break line on one side and '100' debossed on the other side. AUST R 234004.
A blister pack contains 10 tablets or 60 tablets.
* Not all strengths and pack sizes may be available.
Ingredients:
Metoprolol contains 50 mg or 100 mg of metoprolol tartrate as the active ingredient.
The tablets also contain the following in-active ingredients:
- Lactose monohydrate
- Microcrystalline cellulose
- Sodium starch glycollate
- Colloidal anhydrous silica
- Croscarmellose sodium
- Pregelatinised maize starch
- Purified talc
- Magnesium stearate
- Hypromellose
- Macrogol 400
- Titanium dioxide
- Iron oxide - red (50 mg only)
Metoprolol tablets contains sugars as lactose.
Sponsor
Wagner Pharmaceuticals Pty Ltd
6 Albert Street
Preston VIC 3072
Tel: 1800 936 140
This leaflet was last updated in September 2024.
Published by MIMS January 2025

 Adverse effects are ranked under the following frequency estimates: very common: ≥ 10%; common: ≥ 1% and < 10%; uncommon ≥ 0.1% and < 1%; rare: ≥ 0.01% and < 0.1%; very rare: < 0.01%.
Adverse effects are ranked under the following frequency estimates: very common: ≥ 10%; common: ≥ 1% and < 10%; uncommon ≥ 0.1% and < 1%; rare: ≥ 0.01% and < 0.1%; very rare: < 0.01%. Chemical name: Bis [(2RS)-11[4- (2-methoxyethyl)phenoxy]-3-[(1-methylethyl)amino] propan-2-ol](2R,3R)-2,3-dihydroxybutanedioate.
Chemical name: Bis [(2RS)-11[4- (2-methoxyethyl)phenoxy]-3-[(1-methylethyl)amino] propan-2-ol](2R,3R)-2,3-dihydroxybutanedioate.