What is in this leaflet
This leaflet answers some common questions about Metrogyl.
It does not contain all of the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have benefits and risks. Your doctor has weighed the risks of you taking Metrogyl against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, talk to your doctor or pharmacist.
Keep this leaflet with your medicine. You may need to read it again.
What Metrogyl is used for
Metrogyl is used to treat certain infections caused by bacteria and other organisms in different parts of the body.
Metrogyl may also be used to prevent or treat certain infections that may occur during surgery.
Metrogyl is an antibiotic which belongs to a group of medicines called nitroimidazoles. These medicines work by killing or stopping the growth of bacteria and other organisms causing these infections.
Your doctor may have prescribed Metrogyl for another reason.
Ask your doctor if you have any questions about why Metrogyl has been prescribed for you.
Metrogyl is available only with a doctor's prescription.
There is no evidence that this medicine is addictive.
Before you take Metrogyl
When you must not take it
Do not take Metrogyl if you are allergic to medicines containing metronidazole, any other nitroimidazole medicine or any of the ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include skin rash, itching or hives, swelling of the face, lips or tongue which may cause difficulty in swallowing or breathing, wheezing or shortness of breath.
Do not take Metrogyl if:
- you have ever had or are having an active disease of the central nervous system (brain, spinal cord or nerves)
- have evidence of, or history of a blood disorder.
Do not take Metrogyl if the expiry date (Exp.) printed on the pack has passed.
Do not take Metrogyl if the packaging shows signs of tampering or the tablets do not look quite right.
Before you start to take it
Tell your doctor if you are allergic to any other medicines, foods, dyes or preservatives.
Tell your doctor if you are pregnant or plan to become pregnant. Your doctor will discuss the risks and benefits of taking Metrogyl during pregnancy.
Tell your doctor if you are breastfeeding or wish to breastfeed. Like many other medicines Metrogyl passes into breast milk and may harm your baby. Metrogyl is not recommended in breastfeeding.
Tell your doctor if you have any medical conditions, especially the following:
- a blood disorder
- disease of the brain, spinal cord or nerves
- kidney problems
- liver problems
- an inflammatory disease of the small intestines (e.g. Crohn's disease)
- Cockayne syndrome
- you drink alcohol.
Do not drink alcohol during (and for 24 hours after stopping) treatment with Metrogyl.
Your doctor may want to take special care if you have any of these conditions.
If you have not told your doctor or pharmacist about any of the above, tell them before you start taking Metrogyl.
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you buy without a prescription from a pharmacy, supermarket or health food shop.
Some medicines may be affected by Metrogyl, or may affect how well it works. These include:
- warfarin, or other medicines used to prevent blood clots
- disulfiram, a medicine used to treat chronic alcohol dependence
- Medications containing alcohol (ethanol), e.g. some cough syrups
- some anticancer drugs such as carmustine (BCNU), cyclophospamide, 5-fluorouracil or busulfan
- lithium, a medicine used to treat mood swings and some types of depression
- phenytoin, a medicine used to treat convulsions
- phenobarbital (phenobarbitone), medicines used to treat convulsions or sedation
- cimetidine, a medicine used to treat reflux and ulcers
- ciclosporin, a medicine used to prevent organ transplant rejection or to treat immune responses.
You may need different amounts of your medicine or you may need to take different medicines. Your doctor will advise you.
If you are not sure whether you are taking any of these medicines, check with your doctor or pharmacist. Your doctor and pharmacist may have more information on medicines to be careful with or avoid while taking Metrogyl.
How to take Metrogyl
How much to take
Follow all directions given to you by your doctor and pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the box ask your doctor or pharmacist for help.
The dose varies from patient to patient. Your doctor will tell you how many tablets you need to take each day and when to take them. This depends on your condition and whether or not you are taking any other medicines.
How to take Metrogyl
Swallow the tablets whole with a glass of water.
Do not crush or chew the tablets.
Metrogyl tablets, however, can be broken in half if your doctor has prescribed half a tablet.
When to take Metrogyl
Take Metrogyl with or immediately after food. This will lessen the chance of a stomach upset.
If you are taking more than a single dose of Metrogyl, space the doses evenly throughout the day.
If you forget to take Metrogyl
If you are taking more than a single dose of Metrogyl, and it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take the missed dose as soon as you remember, and then go back to taking your tablets as you would normally.
Do not take a double dose to make up for the dose you missed.
If you are not sure what to do, ask your doctor or pharmacist.
If you have any questions about this, check with your doctor or pharmacist.
If you have trouble remembering to take Metrogyl, ask your pharmacist for some hints.
How long to take Metrogyl for
Keep taking Metrogyl until you finish the bottle, or for as long as your doctor recommends.
For treating infections, Metrogyl is usually taken for 7 days, however your doctor may prescribe Metrogyl for longer or shorter periods of time depending on the condition you are being treated for.
Your doctor will tell you how much Metrogyl to take.
Check with your doctor if you are not sure how long you should be taking it for.
If you take too much Metrogyl (overdose)
Immediately telephone your doctor, or the Poisons Information Centre (telephone 13 11 26), or go to Accident and Emergency at the nearest hospital, if you think you or anyone else may have taken too much Metrogyl. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
If you take too much Metrogyl, you may feel disorientated, unsteadiness when walking and vomiting may occur.
While you are taking Metrogyl
Things you must do
Before starting any new medicine, tell your doctor or pharmacist that you are taking Metrogyl.
Tell all the doctors, dentists and pharmacists who are treating you that you are taking Metrogyl.
Make sure you have any blood tests that your doctor requests, especially if you are taking Metrogyl for longer than 10 days or if you are elderly.
If you have to have any other blood tests, tell your doctor that you are taking Metrogyl.
Metrogyl may affect the results of some tests.
If you notice a persistent numbness, tingling or weakness of the arms or legs, stop taking Metrogyl and tell your doctor immediately.
If you become pregnant while taking Metrogyl, stop taking it and tell your doctor immediately.
If you get a sore, white mouth or tongue while taking or soon after stopping Metrogyl treatment, tell your doctor. Also tell your doctor if you get vaginal itching or discharge. This may mean you have a fungal/yeast infection called thrush. Sometimes, the use of Metrogyl allows fungi/yeast to grow and the above symptoms to occur. Metrogyl does not work against fungi/yeast.
Things you must not do
Do not drink any alcohol while taking Metrogyl and for at least 24 hours after stopping treatment. Drinking alcohol may make you feel very sick, vomit, have stomach cramps, headaches and flushing.
Do not stop taking Metrogyl, even if you feel better after a few days, unless advised by your doctor. Your infection may not clear completely if you stop taking your medicine too soon because the bacteria/organism causing your infection may not have been killed. These bacteria/organisms may continue to grow and multiply so that your infection may not improve or it can return again.
Do not use Metrogyl to treat any other conditions unless your doctor tells you to.
Do not give Metrogyl to anyone else, even if they have the same condition as you.
Things to be careful of
Be careful driving or operating machinery until you know how Metrogyl affects you.
Metrogyl may cause confusion, dizziness or hallucinations in some people. If any of these occur, do not drive, operate machinery or do anything else that could be dangerous.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Metrogyl even if you do not think the problem is connected with the medicine or is not listed in this leaflet.
Like all other medicines, Metrogyl may have unwanted side effects in some people. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you notice any of the following and they worry you:
- oral thrush - white, furry sore or inflamed tongue and mouth
- vaginal thrush - sore and itchy vagina with or without discharge
- nausea, which may be accompanied by headache, loss of appetite, and vomiting
- diarrhoea, stomach discomfort, abdominal cramping or constipation, strange taste in mouth
- convulsions, dizziness, weakness, feeling of incoordination or uncoordinated movements
- confusion, irritability, depression or sleeplessness
- skin rashes, flushing, itching
- stuffy nose, dry mouth, nasal congestion, dryness of the mouth (or vagina or vulva)
- unusual urination patterns (e.g. difficulty in passing urine, large amounts of urine, incontinence, or pus in urine)
- joint pain
- eye problems, including blurred or double vision
- hearing problems
- yellowing of the skin or eyes, which may be jaundice
If any of the following happen, stop taking Metrogyl and tell your doctor immediately, or go to Accident and Emergency at the nearest hospital:
- skin rash, itchiness, hives
- swelling of the face, lips, mouth, throat or neck which may cause difficulty swallowing or breathing
- tingling or numbness in the hands or feet, or muscle weakness
- severe upper stomach pain, often with nausea and vomiting.
If you have been on prolonged Metrogyl therapy and experience any unusual numbness of the feet or hands, stop taking Metrogyl, and tell your doctor immediately.
Other side effects not listed above may also occur in some patients.
Tell your doctor if you notice anything that is making you feel unwell.
After taking Metrogyl
Storage
Keep Metrogyl where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Keep your tablets in a cool dry place where the temperature stays below 30°C. Protect from light.
Do not store Metrogyl or any other medicine in the bathroom or near a sink.
Do not leave Metrogyl in the car or on window sills. Heat and dampness can destroy some medicines.
Disposal
If your doctor tells you to stop taking Metrogyl, or your tablets have passed their expiry date, ask your pharmacist what to do with any that are left over.
Product description
What it looks like
Metrogyl comes in 2 strengths of tablets:
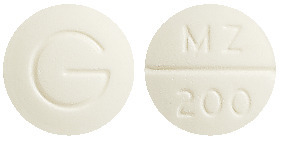
- Metrogyl 200 - round white tablet marked MZ/200 on one side and G on the reverse
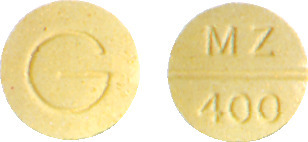
- Metrogyl 400 - round yellow tablet marked MZ/400 on one side and G on the reverse.
Metrogyl 200 and Metrogyl 400 available as 21 tablets.
Metrogyl 200 is also available as 250 tablets (hospital use only).
Ingredients
The active ingredient in Metrogyl is metronidazole.
Each Metrogyl tablet contains either 200 mg or 400 mg of metronidazole.
The tablets also contain:
- lactose monohydrate
- disodium edetate
- ethylcellulose
- sodium starch glycollate
- colloidal anhydrous silica
- guar gum
- magnesium stearate.
Metrogyl 400 also contains quinoline yellow CI 47005 (E104).
The tablets are gluten free.
Metrogyl tablets contain sulfites and sugars (as lactose and galactose).
Sponsor
Metrogyl is supplied in Australia by:
Alphapharm Pty Limited
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.mylan.com.au
Australian registration numbers:
Metrogyl 200 - Aust R 17654
Metrogyl 400 - Aust R 17655
This leaflet was prepared in June 2019.
Metrogyl_cmi\Jun19/00
Published by MIMS September 2019


 Metronidazole is a 1-(2-hydroxyethyl)-2-methyl-5-nitroimidazole.
Metronidazole is a 1-(2-hydroxyethyl)-2-methyl-5-nitroimidazole.