What is in this leaflet
This leaflet answers some common questions about Minipress. It does not contain all the available information.
It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking Minipress against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with your medicine. You may need to read it again.
What Minipress is used for
Minipress is used to treat:
- high blood pressure, also called hypertension
- benign prostatic hyperplasia or BPH ('prostate trouble') in men waiting for prostate surgery
- Raynaud's disease, where the fingers become white and painful when cold
- certain types of heart failure.
When used to treat high blood pressure or heart failure, Minipress is often used in combination with other medicines.
Minipress contains the active ingredient prazosin and belongs to a family of medicines called alpha blockers.
These medicines work by relaxing muscles in the walls of blood vessels and reducing the resistance to blood flow. They also relieve prostate problems by relaxing muscles in the prostate gland and increasing the flow of urine.
Your doctor may have prescribed Minipress for another reason.
Ask your doctor if you have any questions about why Minipress has been prescribed for you.
This medicine is only available with a doctor's prescription.
Before you take Minipress
When you must not take it
Do not take Minipress if you have an allergy to:
- any medicine containing prazosin
- any of the ingredients listed at the end of this leaflet
- any other alpha blocker medicine (e.g. Hytrin).
Ask your pharmacist if you are not sure if you are taking one of these medicines.
Symptoms of an allergic reaction to these medicines may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin.
Do not take Minipress if:
- the packaging is torn or shows signs of tampering
- the expiry date (EXP) printed on the pack has passed.
If you are not sure whether you should be taking Minipress, contact your doctor.
Use in children
Minipress is not recommended for use in children under 12 years of age. Safety in children younger than 12 years has not been established.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you have or have had any medical conditions, especially the following:
- heart problems such as heart failure or angina
- kidney or liver problems.
Tell your doctor if you are pregnant or breastfeeding or plan to become pregnant or breastfeed. Your doctor will discuss the risks and benefits of taking it during pregnancy or while breastfeeding.
If you have not told your doctor about any of the above, tell them before you start Minipress.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you buy without a prescription from a pharmacy, supermarket or health food shop. Some medicines and Minipress may interfere with each other.
Your doctor or pharmacist has a complete list of medicines to be careful with or avoid while taking Minipress.
If you have not told your doctor or pharmacist about these things, tell them before you start taking Minipress.
In particular, tell your doctor if you are taking:
- medicines used to lower blood pressure
- fluid tablets (diuretics)
- medicines to treat impotence (erectile dysfunction).
Other medicines that lower high blood pressure may have an additive effect with Minipress and make your blood pressure too low. As a result, their dose may need to be changed when Minipress is started.
How to take Minipress
Take Minipress exactly as your doctor has prescribed.
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the pack, ask your doctor or pharmacist for help.
How much to take
Minipress is usually started at a low dose of 0.5 mg (half a 1 mg tablet) taken twice a day.
Starting with a low dose reduces the risk of too great a drop in your blood pressure which can make you dizzy, lightheaded or faint.
Your doctor may gradually increase this dose as required. This may depend on your age, your condition and whether or not you are taking any other medicines.
Hypertension (high blood pressure)
The usual starting dose is 0.5 mg (half a 1 mg tablet) taken twice a day increasing to 1 mg taken two or three times a day. Your doctor may increase this up to 20 mg a day, taken in divided doses.
Heart failure
The usual starting dose is 0.5 mg (half a 1 mg tablet) increasing to 4 mg a day, divided into three or four doses. This may be increased by your doctor up to 20 mg a day, taken in divided doses.
Raynaud's disease
The usual starting dose is 0.5 mg (half a 1 mg tablet) taken twice a day. Your doctor may increase this up to 1 mg or 2 mg taken twice a day.
Benign prostatic hyperplasia (BPH)
The usual starting dose is 0.5 mg (half a tablet) taken twice a day. Your doctor may increase this to 2 mg taken twice a day.
When to take it
Take your Minipress tablets at the same time each day. Taking your tablets at about the same time each day will have the best effect. This will also help you remember when to take them.
When you first start taking Minipress or if your doctor increases your dose, take the first dose last thing at night, just before going to bed.
Be especially careful if you need to get up during the night, because you may feel dizzy and could fall.
How to take it
Swallow the tablets with a glass of water or other liquid.
It does not matter if you take Minipress before or after food.
How long to take it
Keep taking Minipress every day until your doctor tells you to stop.
If you are taking Minipress for high blood pressure, heart failure or Raynaud's disease, you may need to take it for a long time.
Minipress will help control these conditions, but will not cure them. Therefore, it must be taken every day.
If you are taking Minipress for prostate problems, you will only have to take it until your operation.
If you forget to take it
If it is almost time for your next dose (e.g. within 3 hours), skip the dose you missed and take the next dose when you are meant to.
Otherwise, take it as soon as you remember, then go back to taking it as you would normally.
Do not take a double dose to make up for the dose you missed.
If you are not sure what to do, check with your doctor or pharmacist.
If you miss two (2) doses or more, you will need to restart at a low dose and build up again gradually to your usual dose.
Ask your doctor how to do this.
If you take too much (Overdose)
Immediately telephone your doctor or Poisons Information Centre (telephone 13 11 26) for advice or go to Accident and Emergency (Casualty) at your nearest hospital if you think that you, a child or anyone else may have taken too much Minipress. Do this even if there are no signs of discomfort or poisoning.
If you take too much Minipress, you may feel lightheaded, dizzy, have a fast or irregular heartbeat, or you may faint.
While you are taking it
Things you must do
Get up slowly after you have been sitting or lying down. Minipress can cause dizziness, lightheadedness and fainting, particularly if you get up too quickly. This is also more likely to occur if you have just started Minipress or the dose of Minipress has just been increased.
These symptoms can be dangerous, particularly if you are 65 years or older and have heart disease.
If you feel dizzy or lightheaded, lie down so that you do not faint. Then sit for a few moments before standing to prevent the dizziness from returning.
If these symptoms continue, tell your doctor. A change in dose may be needed.
See your doctor as soon as possible if you experience painful erections or if your erection continues for longer than four hours. You may need urgent medical attention.
If you become pregnant while taking Minipress, tell your doctor immediately.
If you are about to start any new medicines, tell your doctor and pharmacist that you are taking Minipress.
Tell all doctors, dentists and pharmacists who are treating you that you are taking Minipress.
Things you must not do
Do not give Minipress to anyone else, even if they have the same condition as you.
Do not use Minipress to treat any other complaints unless your doctor tells you to.
Do not stop taking Minipress, or lower the dosage, without checking with your doctor. Your doctor will reduce your dose of Minipress gradually if you are to stop taking this medicine.
Things to be careful of
Be careful driving or operating machinery until you know how Minipress affects you. Minipress may cause dizziness, lightheadedness or fainting in some people, especially after the first dose or after a dose increase. Blurred vision or drowsiness may also occur.
Make sure you know how you react to Minipress before you drive a car, operate machinery or do anything else that could be dangerous if you are dizzy, drowsy, or are not alert.
Be careful to limit the amount of alcohol you drink while taking Minipress. Also, take extra care during exercise or hot weather or if you have to stand for a long time. Dizziness, lightheadedness, or fainting is more likely to occur if you drink alcohol, stand for a long time, exercise or the weather is hot.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Minipress.
Minipress helps most people but it may have some unwanted side effects in a few people. All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Ask your doctor or pharmacist to answer any questions you may have.
If you are 65 years or older, you should be especially careful while taking Minipress. Report any side effects promptly to your doctor.
Following is a list of possible side effects. Do not be alarmed by this list. You may not experience any of them.
Tell your doctor if you notice any of the following and they worry you:
- feeling sick, vomiting or dry mouth
- constipation, diarrhoea
- weakness or lack of energy
- headache or drowsiness
- stuffy nose.
These side effects are usually mild.
Tell your doctor immediately if you notice any of the following:
- fast or pounding heartbeat, chest pain,
- fainting, dizziness or lightheadedness when standing up
- shortness of breath or difficulty breathing
- blurred vision
- rash, itching or other skin problems
- sharp pain in the stomach or back
- persistent painful erection of the penis which occurs without sexual arousal
- tingling or numbness of the hands or feet
- swelling of the hands, feet or ankles
- feelings of nervousness or depression.
These may be serious side effects. You may need urgent medical attention.
This is not a complete list of all possible side effects. Others may occur in some people and there may be some side effects not yet known.
If you notice any other symptoms that worry you, check with your doctor.
Ask your doctor or pharmacist if you don't understand anything in this list.
After taking Minipress
Storage
Keep your tablets in their pack until it is time to take them. If you take them out of their packaging, they will not keep well.
Keep them in a cool, dry place where the temperature stays below 30°C. Do not store them, or any other medicine in the bathroom or near a sink.
Do not leave them in the car or on window sills. Heat and dampness can destroy some medicines.
Keep your tablets where young children cannot reach them. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking Minipress, or the tablets have passed their expiry date, ask your pharmacist what to do with any left over.
Product description
What it looks like
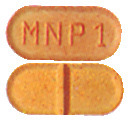
- Minipress 1mg - orange, capsule-shaped tablets marked MNP 1 on one side and scored on the other.
- Minipress 2mg - white, round tablets marked MNP 2 on the scored side and plain on the other.
- Minipress 5mg - white, diamond shaped tablets scored on both sides, marked MNP 5 on one side.
Each box contains 100 tablets.
Ingredients
Minipress tablets contain either 1 mg, 2 mg or 5 mg of prazosin as the active ingredient.
The other ingredients are:
- maize starch
- microcrystalline cellulose
- magnesium stearate
- sodium lauryl sulfate
- calcium hydrogen phosphate
- sunset yellow FCF (1 mg tablet only).
Minipress does not contain lactose, or sucrose.
Manufacturer
Minipress is supplied in Australia by:
Pfizer Australia Pty Ltd
Sydney NSW
Toll Free Number: 1800 675 229
www.pfizer.com.au
Australian Registration Numbers:
Minipress 1mg - AUST R 10756
Minipress 2mg - AUST R 10757
Minipress 5mg - AUST R 10758
This leaflet was prepared in November 2019
® = Registration Trademark
© Pfizer Australia Pty Ltd
Published by MIMS February 2020