What is in this leaflet
This leaflet answers some common questions about Mogadon. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking Mogadon against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What Mogadon is used for
Mogadon is used to treat sleeping problems, also called insomnia.
Mogadon contains the active ingredient nitrazepam, a benzodiazepine. It is thought to work by its action on brain chemicals.
In general, benzodiazepines such as Mogadon should be taken for short periods only (for example 2 4 weeks). Continuous long-term use is not recommended unless advised by your doctor. The use of benzodiazepines may lead to dependence on the medicine.
This medicine is available only with a doctor's prescription.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another purpose.
Before you take Mogadon
When you must not take it
- Do not take Mogadon if you have an allergy to:
- nitrazepam or any of the ingredients listed at the end of this leaflet
- any other medicine from the benzodiazepine group of medicines such as diazepam, temazepam, oxazepam, alprazolam and clonazepam.
Some of the symptoms of an allergic reaction may include skin rash, itching or hives.
- You have severe and chronic lung disease (e.g. chronic obstructive airway disease) and have difficulty breathing.
- You have a severe liver disorder.
Do not take the tablets if the packaging is torn or shows signs of tampering.
Do not take the medicine after the expiry date (EXP) printed on the pack. If you take it after the expiry date has passed, it may not work as well.
Do not give this medicine to children unless advised by the child's doctor. The safety and effectiveness of this medicine in children have not been established.
Before you start to take it
You must tell your doctor if:
- You have allergies to
- any other medicines
- any other substances, such as foods, preservatives or dyes
- You are pregnant or plan to become pregnant.
Your doctor will discuss the risks and benefits of taking this medicine during pregnancy.
- You are breastfeeding or plan to breastfeed.
Your doctor will discuss the risks and benefits of taking this medicine when breastfeeding.
- You have or have had any other medical conditions including:
- liver, kidney or lung disease
- if you suffer from fits or convulsions
- if you suffer from severe muscle weakness known as myasthenia gravis
- if you have high or low blood pressure
- if you have glaucoma (high pressure in the eye)
- if you suffer from depression, psychosis or schizophrenia
- You drink alcohol regularly.
Alcohol may increase the effects of this medicine.
- You have a history of falling or are unsteady when walking.
If you have not told your doctor about any of the above, tell them before you take any Mogadon.
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interfere with each other. These include:
- other sleeping tablets, sedatives or tranquillisers
- medicines for depression
- medicines for allergies for example antihistamines or cold tablets
- pain relievers
- muscle relaxants
- medicines to control fits
- cimetidine, a medicine used to treat ulcers and reflux
- disulfiram, a medicine used to deter the consumption of alcohol.
These medicines may increase the effects of Mogadon or be affected by it. You may need to take different amounts of your medicine or you may need to take different medicines. Your doctor will advise you.
Your doctor or pharmacist may have more information on medicines to be careful with or avoid while taking Mogadon.
How to take Mogadon
How much to take
The dose of Mogadon may be different for each person. Your doctor will decide the right dose for you.
The usual adult dose of Mogadon is between 5 to 10 mg (one to two tablets).
For the elderly, the usual dose of Mogadon is 2.5 mg to 5 mg (half to one tablet).
How to take it
Swallow Mogadon whole with a full glass of water.
When to take it
Take the tablet before going to bed.
How long to take it
Do not use Mogadon for longer than your doctor says.
Mogadon is usually taken for short periods only (for example 2 - 4 weeks). Continuous long-term use is not recommended unless advised by your doctor. The use of benzodiazepines may lead to dependence on the medicine.
If you forget to take it
If you forget to take Mogadon before you go to bed and you wake up late in the night or early morning, do not take any Mogadon as you may have trouble waking in the morning. If you have any questions about this, ask your doctor or pharmacist.
If you have trouble remembering when to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much Mogadon. Do this even if there are no signs of discomfort or poisoning. Report any other medicine or alcohol, which has been taken. You may need urgent medical attention. Keep telephone numbers for these places handy.
If you take too much Mogadon the mild symptoms are drowsiness, mental confusion and lethargy. In more serious cases, symptoms are inability to move, loss of muscle tone, hypotension, breathing difficulties, coma and very rarely death.
While you are taking Mogadon
Things you must do
Use Mogadon exactly as your doctor has prescribed.
Tell all doctors, dentists and pharmacists who are treating you that you are taking Mogadon especially if you are about to be started on any new medicines.
If you become pregnant while you are taking Mogadon, tell your doctor immediately.
Tell your doctor if you feel Mogadon is not helping your condition.
Visit your doctor regularly. Your doctor needs to check your progress and see whether you need to keep taking Mogadon.
Always discuss with your doctor any problems or difficulties during or after taking Mogadon.
Tell your doctor if, for any reason, you have not taken your medicine exactly as prescribed. Otherwise your doctor may think that it was not effective and change your treatment unnecessarily.
Keep enough Mogadon to last weekends and holidays.
Things you must not do
Do not drive or operate machinery until you know how Mogadon affects you. Mogadon causes drowsiness and affects alertness in most people.
Make sure you know how you react to Mogadon before you drive a car, operate machinery, or do anything else that could be dangerous if you are drowsy, dizzy or not alert.
Even if you take Mogadon at night, you may still be drowsy or dizzy the next day.
Do not take Mogadon for a longer time than your doctor has prescribed.
Do not stop taking Mogadon or change your dose, without first checking with your doctor. Stopping this medicine suddenly may cause some unwanted effects. Your doctor will slowly reduce your dose of Mogadon before you can stop taking it completely.
Do not suddenly stop taking Mogadon if you suffer from epilepsy. Stopping this medicine suddenly may make your epilepsy worse.
Do not use this medicine to treat any other complaints unless your doctor says to.
Do not give Mogadon to anyone else, even if their symptoms seem similar to yours.
Things to be careful of
Be careful when drinking alcohol while you are taking Mogadon. Combining Mogadon and alcohol can make you more sleepy, dizzy, lightheaded or increase the risk of sleep disorders.
Your doctor may suggest that you avoid alcohol or reduce the amount of alcohol you drink while you are taking Mogadon.
You should not take Mogadon if you experience complex sleep behaviours such as sleep walking, sleep driving or any other bizarre sleep-related behaviours.
Be careful if you are elderly, unwell or taking other medicines. Some people may experience side effects such as drowsiness, confusion, dizziness and unsteadiness. These may increase the risk of a fall.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are using Mogadon. Mogadon helps most people with insomnia, but it may have unwanted side effects in some people.
All medicines may have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you notice any of the following and they worry you:
- drowsiness
- dizziness
- fatigue
- confusion
- unsteadiness when walking
- impairment of memory
- headache
- hangover feeling in the morning
- slurred speech
- clumsiness, lack of coordination, numbed emotions
- reduced alertness
- muscle weakness
- double vision
- inattention
- unpleasant dreams
- rebound insomnia.
These side effects are usually mild.
Tell your doctor immediately, or go to Accident and Emergency at your nearest hospital, if you notice any of the following:
- swelling of the tongue or throat
- difficulty in breathing.
Tell your doctor if you notice anything else that is making you feel unwell when you are taking, or soon after you have finished taking Mogadon. Other side effects not listed above may occur in some patients.
Like other medicines, Mogadon can cause some side effects. If they occur, they are most likely to be minor and temporary. However, some may be serious, such as complex sleep behaviours, and need medical attention.
Tell your doctor if you notice any unusual changes in your sleep behaviour. Some people may get other side effects while using Mogadon.
Ask your doctor or pharmacist if you don't understand anything in this list.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
After using Mogadon
Storage
Keep your tablets in their blister pack until it is time to take them. If you take the tablets out of the blister pack they may not keep well.
Keep Mogadon in a cool dry place where the temperature stays below 30°C. Protect from light. Do not store it or any other medicine in the bathroom or near a sink. Do not leave it in the car or on window sills. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking Mogadon or the tablets have passed their expiry date, ask your pharmacist what to do with any tablets left over.
Product description
What Mogadon looks like
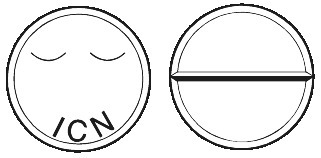
Mogadon tablets are white, marked ICN on the upper face and a single break bar on the lower face.
Mogadon comes in a blister pack containing 25 tablets.
Ingredients
Mogadon contains 5mg nitrazepam. Mogadon also contains lactose, starch - maize and magnesium stearate.
Mogadon does not contain gluten, sucrose, tartrazine or any other azo dyes.
Supplier
iNova Pharmaceuticals (Australia) Pty Limited
ABN: 13 617 871 539
Level 10, 12 Help Street
Chatswood NSW 2067
Tel: 1800 630 056
®= Registered Trademark
AUST R 13751
Date of last amendment: December 2017.
Published by MIMS February 2018