What is in this leaflet
This leaflet answers some of the common questions people ask about NEXIUM Hp7. It does not contain all the available information about NEXIUM Hp7.
It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor will have weighed the risks of you taking NEXIUM Hp7 against the benefits they expect it will have for you.
If you have any concerns about taking these medicines, ask your doctor or pharmacist.
Keep this leaflet with the medicines. You may need to read it again.
What NEXIUM Hp7 is used for
NEXIUM Hp7 is a combination pack, which contains three different medicines. When taken together in the right doses, they will kill the bacteria in your stomach called Helicobacter pylori and let your peptic ulcer heal.
Depending on the position of the ulcer it is called a gastric or duodenal ulcer. A gastric ulcer occurs in the stomach. A duodenal ulcer occurs in the duodenum which is the tube leading out from the stomach.
Most people who have a peptic ulcer also have a bacterium called Helicobacter pylori in their stomach.
If the bacteria are killed it is unlikely that your ulcer will come back.
NEXIUM is the brand name of esomeprazole (E), and it is given with amoxicillin (A), brand name Amoxil and clarithromycin (C), brand name Klacid. Amoxil and Klacid are both types of antibiotic.
How NEXIUM Hp7 works
NEXIUM is a type of medicine called a proton-pump inhibitor.
NEXIUM works by decreasing the amount of acid made by the stomach, to give relief of symptoms and allow healing to take place. This does not stop food being digested in the normal way.
Amoxicillin and clarithromycin are both antibiotics that help kill Helicobacter pylori. NEXIUM also helps kill the bacteria. When all three are taken together they are more effective than taken one or two at a time. It is possible that the antibiotics may not always kill Helicobacter pylori.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may prescribe this medicine for another use.
There is no evidence that NEXIUM Hp7 is addictive.
This medicine is available only with a doctor's prescription.
Before you take NEXIUM Hp7
When you must not take it
Do not take NEXIUM Hp7 if you have an allergy to:
- any medicines containing esomeprazole, amoxicillin or clarithromycin
- any ingredients listed at the end of the leaflet
- any other similar medicines such as proton pump inhibitors, penicillins, cephalosporins or macrolide antibiotics
Some of the symptoms of an allergic reaction may include shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue or other parts of the body; rash, itching or hives on the skin.
Do not take NEXIUM Hp7 if you are taking any of the following medicines:
- ergotamine
- dihydroergotamine
- cisapride
- pimozide
- astemizole
- terfenadine
- cilostazol
- atazanavir
- colchicine
- simvastatin
- lovastatin
- ticagrelor
- ranolazine
- oral midazolam
- domperidone
- lomitapide
Please check with your doctor or pharmacist if you are taking any of these medicines. These medicines will be affected by the medicines in NEXIUM Hp7 and it is more likely you will get side effects.
Do not take NEXIUM Hp7 if you have a history of heart conditions such as QT prolongation or ventricular cardiac arrhythmia, including torsades de pointes.
Do not take NEXIUM Hp7 if you have low magnesium or potassium levels.
Do not take NEXIUM Hp7 if you have both liver and kidney problems.
NEXIUM Hp7 is not recommended for use in children. There is no information about the use of NEXIUM Hp7 in children.
Do not take this medicine after the use by (expiry) date printed on the pack or if the packaging is torn or shows signs of tampering If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to use it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
You must tell your doctor if you have:
- problems with your blood
- problems with your liver or kidneys
- any heart conditions, such as QT prolongation or ventricular cardiac arrhythmia
- you have glandular fever
- myasthenia gravis, a condition in which the muscles become weak and tire easily
- been diagnosed with osteoporosis
- low potassium, low magnesium or any other electrolyte imbalances
- if you have ever had a skin reaction after treatment with a medicine similar to NEXIUM that reduces stomach acid
Tell your doctor if you are pregnant or plan to be pregnant, are breast-feeding or plan to breast-feed. It is not known if it is safe for you to take NEXIUM Hp7 if you are pregnant. The medicines in NEXIUM Hp7 may affect the developing baby.
Your baby can take in all the medicines in NEXIUM Hp7 from breast milk if you are breast-feeding. NEXIUM Hp7 is not recommended when breast-feeding.
Your doctor can discuss with you the risks and benefits of using NEXIUM Hp7 if you are pregnant or breast-feeding.
If you have not told your doctor about any of the above, tell them before you start taking NEXIUM Hp7.
Taking other medicines
Do not take NEXIUM Hp7 if you are taking the following medicines:
- ergotamine or dihydroergotamine - medicines used to treat migraine headaches
- astemizole or terfenadine - medicines used to treat hayfever and allergies
- atazanavir - a medicine used to treat Human Immunodeficiency Virus (HIV)
- cilostazol - a medicine used to treat intermittent claudication
- colchicine - a medicine used to treat gout
- lovastatin or simvastatin (statins) - medicines used to treat high cholesterol
- ticagrelor - a medicine used to treat blood clots
- oral midazolam - a medicine used for surgical sedation
- domperidone - a medicine used for nausea
- ranolazine - a medicine used to treat angina (chest pain)
- cisapride - a medicine used to treat gastric reflux
- pimozide - a medicine used to treat psychiatric conditions, such as schizophrenia
- lomitapide - a medicine used to treat high cholesterol
Tell your doctor if you are taking any other medicines, including any that you buy without a prescription from a pharmacy, supermarket or health food shop.
Some medicines and NEXIUM Hp7 may interfere with each other. These include:
- diazepam, triazolam or alprazolam - medicines used as sedatives or to treat anxiety
- ketoconazole, itraconazole, voriconazole or fluconazole - medicines used to treat fungal infections
- warfarin, apixaban, rivaroxaban, dabigatran and clopidogrel - medicines used to prevent blood clots
- allopurinol or probenecid - medicines used to treat gout
- methotrexate, a medicine used to treat arthritis and some types of cancers
- phenytoin, sodium valproate or carbamazepine - medicines used to treat seizures
- atorvastatin or rosuvastatin (statins) - medicines used to treat high cholesterol
- theophylline - a medicine used to treat asthma
- zidovudine, saquinavir, efavirenz, etravirine, nevirapine, nelfinavir or ritonavir - medicines used to treat HIV
- insulin, repaglinide, nateglinide, pioglitazone or rosiglitazone - medicines used to treat diabetes
- digoxin, quinidine or disopyramide - medicines used to treat heart conditions
- citalopram, fluoxetine, clomipramine and imipramine - medicines used to treat depression
- St John's wort - a herbal remedy used to treat mood disorders
- rifabutin, rifapentine, rifampicin or erythromycin - medicines used to treat bacterial infections
- sildenafil, tadalafil or vardenafil - medicines used to treat impotence
- tolterodine - a medicine used to treat incontinence
- verapamil, amlodipine or diltiazem - medicines used to treat high blood pressure and some heart conditions
- methylprednisolone - a corticosteroid
- vinblastine - a medicine used to treat cancer
- oral contraceptives or birth control pills. Talk to your doctor about the need for an additional method of contraception while on NEXIUM Hp7
- tacrolimus or ciclosporin - medicines used to prevent organ transplant rejection or to treat certain problems with the immune system
- methotrexate - a medicine used to treat arthritis and some types of cancer
- erlotinib or related medicines used to treat cancer
- phenobarbitone - a medicine used to treat epilepsy
- tetracycline and aminoglycoside antibiotics - medicines used to treat certain infections
- atypical antipsychotics - medicines used to treat psychiatric conditions, such as schizophrenia and bipolar disorder (e.g. quetiapine)
- ibrutinib - a medicine used to treat cancer
These medicines may affect NEXIUM Hp7 or may affect how well it works. You may need different amounts of your medicines or you may need to take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
If you have not told your doctor about any of these things, tell them before you take any NEXIUM Hp7.
How to take NEXIUM Hp7
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the box, ask your doctor or pharmacist for help.
How much to take
There are three different medicines in NEXIUM Hp7. It is very important that you take NEXIUM Hp7 exactly as follows:
NEXIUM (esomeprazole) = E
Take one 20 mg tablet in the morning and one 20 mg tablet at night.
Amoxil (amoxicillin) = A
Take two 500 mg capsules in the morning and two 500 mg capsules at night.
Klacid (clarithromycin) = C
Take one 500 mg tablet in the morning and one 500 mg tablet at night.
Morning
1 E, 2 A, 1 C
Night
1 E, 2 A, 1 C
How to take it
Swallow the NEXIUM tablets whole with a glass of water. For patients with swallowing difficulties, the tablet can be placed in half a glass of non-carbonated water (mineral water is not suitable). The tablet may be gently mixed and care should be taken not to crush the tablet. Rinse the glass with half a glass of fluid and drink.
NEXIUM tablets may also be administered, dispersed in non-carbonated water, through a gastric tube.
Do not crush or chew the tablets as they will not work properly.
Take medicines in NEXIUM Hp7 during or after meals.
How long to take it
Continue taking the capsules and tablets until you finish the course or until your doctor tells you to stop. If you do not complete the full course prescribed by your doctor, the Helicobacter pylori may not clear completely and your symptoms may return.
Tell your doctor if your symptoms return. It is possible that the antibiotics may not kill Helicobacter pylori. You may need treatment with more antibiotics.
If you forget to take it
If you forget to take any of the medicines in NEXIUM Hp7 take it as soon as you remember, as long as it is more than four hours before the next dose of that medicine is due.
Do not take a double dose to make up for any dose that you miss.
If you are unsure what to do ask your doctor or pharmacist.
If you have trouble remembering when to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor, the Poisons Information Centre (13 11 26) or go to Accident and Emergency at your nearest hospital if you think that you or anyone else may have taken too much NEXIUM Hp7 even if there are no signs of discomfort or poisoning.
If you take too much NEXIUM Hp7 you may vomit and have severe stomach problems.
While you are using NEXIUM Hp7
Things you must do
Take NEXIUM Hp7 exactly as your doctor has prescribed.
If you are about to be started on any new medicine, tell your doctor and pharmacist that you are taking NEXIUM Hp7.
If you become pregnant while you are taking NEXIUM Hp7, tell your doctor.
If you get severe diarrhoea, tell your doctor, pharmacist or nurse immediately. Do this even if it occurs several weeks after NEXIUM Hp7 has been stopped. Diarrhoea may mean that you have a serious condition affecting your bowel. You may need urgent medical care. Do not take medicine to stop the diarrhoea without first checking with your doctor.
If you get a sore, white mouth or tongue while taking, or soon after stopping NEXIUM Hp7, tell your doctor. Also tell your doctor if you get vaginal itching or discharge. This may mean you have a fungal infection called thrush. Sometimes the use of NEXIUM Hp7 allows fungi to grow and the above symptoms to occur. NEXIUM Hp7 does not work against fungal infections.
Tell any other doctors, dentists and pharmacists who are treating you that you are taking NEXIUM Hp7.
If you need to have any medical tests while you are taking NEXIUM Hp7, tell your doctor. It may affect the results of some tests.
Drink plenty of water or fluids while taking NEXIUM Hp7
Things you must not do
Do not take NEXIUM Hp7 to treat any other complaints unless your doctor tells you to.
Do not stop taking NEXIUM Hp7, or change any of the doses, unless you have discussed it with your doctor. You need to take all the capsules and tablets in the pack for it to work properly. If you stop taking it, your ulcer may come back and be harder to treat next time.
Do not give NEXIUM Hp7 to anyone else, even if they have the same condition as you.
Things to be careful of
Be careful driving or operating machinery until you know how NEXIUM Hp7 affects you.
NEXIUM Hp7 may cause dizziness in some people. Make sure you know how you react to NEXIUM Hp7 before you do anything that may be dangerous if you are dizzy.
Please talk to your doctor or pharmacist about these possibilities if you think they may bother you.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking NEXIUM Hp7.
NEXIUM Hp7 helps most people with peptic ulcer and Helicobacter pylori infection, but it may have unwanted side effects in a few people.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you notice any of the following and they worry you:
- diarrhoea, nausea or vomiting
- headache
- dizziness
- constipation
- loss of appetite
- stomach pain (e.g. cramps)
- wind
- dryness of the mouth or other body cavities
- soreness of mouth, throat or tongue
- "pins and needles"
- metallic taste or other change in taste or smell
- chills
- fatigue
- excessive burping
These side effects are usually mild.
Tell your doctor immediately or go to Accident and Emergency at your nearest hospital if you notice the following:
- muscle pain or weakness
- increase in breast size (males)
- fever
- changes in sleep patterns
- mood changes, hallucinations, confusion or depression
- change in sexual function
- blurred vision, hearing disturbances
- hair loss
- bleeding or bruising more easily than normal
- signs of frequent infections such as fever, severe chills, sore throat or mouth ulcers
- skin rash
- blood in urine
- tremor
- convulsions or fits
- overgrowth of yeast infections (thrush)
- fainting
- yellowing of the eyes or skin
- muscle twitching or jerking movements
- irregular (fast or slow) heartbeat
- loss of consciousness or awareness
These may be serious side effects. You may need urgent medical attention.
If any of the following happen, stop taking NEXIUM Hp7 and tell your doctor immediately or go to Accident and Emergency at your nearest hospital:
- shortness of breath, wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- chest pain
- severe skin reaction which may include rash, itching, redness, blistering or peeling of the skin
- severe upper stomach pain, with nausea and vomiting
- signs of liver inflammation including yellowing of the skin or eyes, feeling generally unwell, nausea, vomiting, loss of appetite
- skin reaction, especially in sun-exposed areas, with joint pain
- headache, increased sensitivity to light, neck stiffness, unsteadiness, fits
- severe stomach or abdominal cramps, watery and severe diarrhoea, which may also be bloody (this may occur several weeks after you stop taking NEXIUM Hp7)
Do not take any medicine to stop the diarrhoea unless advised by your doctor.
These are very serious side effects. If you have them, you may have had a serious reaction to one of the medicines in NEXIUM Hp7. You may need urgent medical attention or hospitalisation.
Tell your doctor if you notice anything else that is making you feel unwell. Some people may get other side effects while taking NEXIUM Hp7.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
After using NEXIUM Hp7
Storage
Keep the three medicines in NEXIUM Hp7 in their separate blisters until it is time to take them.
Keep it in a cool dry place where the temperature stays below 25°C.
Do not store it or any other medicine in the bathroom or near a sink.
Do not leave it in the car on hot days. Heat and dampness can destroy some medicines.
Keep it where young children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking them, or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What NEXIUM Hp7 looks like
NEXIUM tablets 20 mg are light pink, oblong shaped, marked with '20 mg' on one side and 'A/EH' on the other side.
AMOXIL capsules 500 mg are red and yellow capsules, marked with 'AM500'.
KLACID tablets 500 mg are pale yellow, oval, film coated tablets.
Ingredients
Each NEXIUM tablet contains esomeprazole 20 mg as the active ingredient, plus:
- glyceryl monostearate
- hyprolose
- hypromellose
- magnesium stearate
- methacrylic acid copolymer
- microcrystalline cellulose
- synthetic paraffin
- macrogol 6000
- polysorbate 80
- crospovidone
- sodium stearylfumarate
- purified talc
- triethyl citrate
- sugar spheres (maize starch and sucrose)
NEXIUM is provided in a blister packs of 14 tablets.
The tablets are coloured with titanium dioxide, iron oxide red and iron oxide yellow.
Each AMOXIL capsule contains amoxicillin trihydrate 500 mg, plus:
- magnesium stearate
- purified talc
- gelatin
AMOXIL is provided in blister packs of 28 capsules.
The hard gelatin capsule is coloured with titanium dioxide, iron oxide yellow, erythrosine, indigo carmine and Tek Product- Tek Print SW-0012 White Ink.
Each KLACID tablet contains clarithromycin 500 mg as the active ingredient, plus:
- croscarmellose sodium
- microcrystalline cellulose
- povidone
- silicon dioxide
- hyprolose
- hypromellose
- purified talc
- sorbitan mono-oleate
- stearic acid
- magnesium stearate
- propylene glycol
- sorbic acid
- vanillin
- titanium dioxide
- quinoline yellow
KLACID is provided in blister packs of 14 tablets. Contains sorbates.
NEXIUM Hp7 does not contain gluten.
Sponsors
AstraZeneca Pty Ltd
ABN 54 009 682 311
66 Talavera Road
Macquarie Park NSW 2113
Telephone: 1800 805 342
Aspen Pharmacare Australia Pty Ltd
34-36 Chandos Street
St Leonards NSW 2065
Viatris Pty Ltd
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
Australian Registration Number:
NEXIUM Hp7 - 281690
This leaflet was prepared in August 2023.
NEXIUM is a registered trade mark of the AstraZeneca group of companies.
AMOXIL is a registered trade mark of the Aspen Global Inc. group of companies.
KLACID is a registered trade mark of Viatris Pty Ltd.
Doc ID-005244781 V1.0
Published by MIMS January 2024



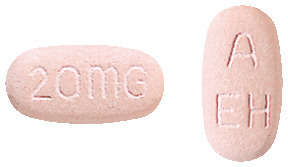

 Adverse reactions that have been observed for the racemate (omeprazole) may occur with esomeprazole.
Adverse reactions that have been observed for the racemate (omeprazole) may occur with esomeprazole. There have been post-marketing reports of colchicine toxicity with concomitant use of clarithromycin and colchicine, especially in the elderly, some of which occurred in patients with renal insufficiency. Deaths have been reported in some such patients (see Section 4.4 Special Warnings and Precautions for Use; Section 4.5 Interactions with Other Medicines and Other Forms of Interactions).
There have been post-marketing reports of colchicine toxicity with concomitant use of clarithromycin and colchicine, especially in the elderly, some of which occurred in patients with renal insufficiency. Deaths have been reported in some such patients (see Section 4.4 Special Warnings and Precautions for Use; Section 4.5 Interactions with Other Medicines and Other Forms of Interactions). In vivo results demonstrate that acid control with esomeprazole is dose dependent and that it is significantly greater, more sustained and less variable compared to an equal dose of the racemate.
In vivo results demonstrate that acid control with esomeprazole is dose dependent and that it is significantly greater, more sustained and less variable compared to an equal dose of the racemate.


