What is in this leaflet
This leaflet answers some common questions about NORFLEX tablets. However it does not contain all the available information. Your doctor and pharmacist have more information about this medicine.
If you have any questions about NORFLEX that are not answered by this leaflet, please ask your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of your taking NORFLEX against the benefits before prescribing it for you.
If you are worried about taking this medicine please talk to your doctor or pharmacist.
Keep this leaflet with your medicine as you may want to read it again.
What NORFLEX is used for
The name of your medicine is NORFLEX. It contains orphenadrine citrate. Orphenadrine citrate is a skeletal muscle relaxant. It acts in the central nervous system to produce its muscle relaxant effects.
NORFLEX is used to help relax certain muscles in your body and to relieve the pain and discomfort caused by sprains, strains or other injury to your muscles.
Your doctor may have prescribed NORFLEX for another purpose.
Ask your doctor if you have any questions about why this medicine has been prescribed for you.
Before you take NORFLEX
When you must not take it
Do not take NORFLEX if:
- you have had an allergic reaction to orphenadrine citrate or other similar medicines, or to any of the ingredients listed at the end of this leaflet.
- you have any of the following:
- glaucoma
(high pressure in the eye);
- intestinal blockage, stomach ulcer or oesophageal disease;
- enlarged prostate or bladder obstruction;
- myasthenia gravis
(a disease of the muscles causing drooping eyelids, double vision, difficulty in speaking and swallowing, and sometimes muscle weakness in the arms or legs). - after the expiry date on the bottle
- if the packaging shows signs of tampering
Do not give this medicine to a child under the age of 12 years. Safety and effectiveness in children younger than 12 years have not been established.
Before you start to take it
Tell your doctor or pharmacist if
- you have allergies to any other medicines, foods, preservatives or dyes.
- you have any heart problems.
- you have any liver or kidney problems.
Tell your doctor if you are pregnant or intend to become pregnant or you are breast-feeding or plan to breast-feed. Your doctor or pharmacist can discuss the risks and benefits involved.
If you are not sure whether to start taking this medicine, talk to your doctor.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you buy without a prescription from your pharmacy, supermarket of health-food shop.
Some medicines may be affected by NORFLEX. These include:
- anticholinergics (medicines used to relieve stomach cramps or spasms)
- medicines used to treat depression
- dextropropoxyphene
- other central nervous system depressant drugs, including alcohol. Examples of CNS depressants are antihistamines or medicines for hayfever, other allergies or colds; sedatives or tranquilizers; narcotic analgesics; barbiturates; medicines for seizures; anaesthetics, including some dental anaesthetics; and other muscle relaxants.
You may need different amounts of your medicines, or you may need to take different medicines. Your doctor or pharmacist has more information on medicines to be careful with or avoid while taking NORFLEX.
How to take NORFLEX
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the bottle ask your doctor or pharmacist for help.
How much to take
The usual dose of NORFLEX in adults is one tablet twice a day. Your doctor will prescribe the correct dose for you.
Take NORFLEX tablets only as directed by your doctor or pharmacist. Do not change the dose unless your doctor tells you to do so.
If you forget to take your medicine
If you miss a dose of this medicine and remember within an hour or so of the missed dose, take it right away. If you do not remember until later, skip the missed dose and go back to taking it as you would normally.
Do not double doses.
If you take too much (overdose)
Do not take more than the recommended dose unless your doctor tells you to. If you or someone else has taken too much NORFLEX contact your doctor or pharmacist, or the Poisons Information Centre (in Australia call 13 11 26; in New Zealand call 0800 POISON or 0800 764 766). Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
Symptoms of an overdose include excitement; confusion; severe confusion leading to coma; convulsions; fast heart rate; dilated pupils and difficulty passing urine.
While you are taking NORFLEX
Things you must do
Tell all doctors, dentists and pharmacists who are treating you that you are taking NORFLEX.
If you become pregnant while taking this medicine tell your doctor immediately.
Be careful driving or operating machinery until you know how the medicine affects you. NORFLEX may cause some people to have blurred vision or to become drowsy, dizzy, light-headed, faint, or less alert than they are normally. It may also cause muscle weakness in some people. If you have any of these symptoms do not drive or operate machinery or do anything else that could be dangerous.
NORFLEX may cause dryness of the mouth. For temporary relief use sugarless gum or sweets, melt some ice in your mouth or use a saliva substitute.
If dry mouth continues for more than 2 weeks speak to your dentist. Continued dryness of the mouth can increase the chance of dental disease, including tooth decay, gum disease and fungal infections.
Things you must not do
NORFLEX is a prescription medicine and has been prescribed for you only.
Do not give it to anyone else even if they have the same condition as you.
Do not take NORFLEX to treat any other complaints unless your doctor tells you to.
Side Effects
All medicines can cause unwanted side effects. Sometimes they are serious, most of the time they are not.
Speak to your doctor if any of the following side effects occur:
LESS COMMON: decreased urination; eye pain; fainting; fast or pounding heartbeat.
RARE: hallucinations; shortness of breath; troubled breathing; tightness in chest and/or wheezing; skin rash; sores, ulcers or white spots on lips or in mouth; swollen and/or painful glands; unusual bruising or bleeding; unusual tiredness or weakness.
The following side effects usually do not need medical attention and may go away during treatment as your body adjusts to the medicine.
Speak to your doctor if any of the following side effects continue or are bothersome:
MORE COMMON: dry mouth.
LESS COMMON OR RARE: abdominal or stomach cramps or pain; blurred or double vision; confusion; constipation; difficulty in urination; dizziness or light-headedness; drowsiness; excitement, irritability, nervousness or restlessness; headache; muscle weakness; unusually large pupils of the eyes.
Other side effects not listed above may also occur in some patients.
If you notice any side effects that are not listed here tell your doctor or pharmacist.
After taking NORFLEX
Storage
Keep this medicine in a cool dry place where the temperature stays below 30°C. Do not store it or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Keep all medicines where children cannot reach them.
A locked cupboard at least 1.5 metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine, or if it has passed its 'Use By' date ask your pharmacist what to do with any tablets that are left over.
Product Description
What it looks like
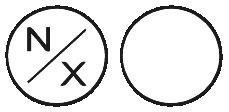
NORFLEX are white tablets with N/X on one side and no markings on the other side, in bottles containing 100 tablets.
Ingredients
Each NORFLEX tablet contains 100mg orphenadrine citrate.
It also contains:
- Magnesium Stearate
- Colloidal Anhydrous Silica
- Ethylcellulose
- Lactose
This medicine does not contain gluten.
Sponsor
NORFLEX is supplied in Australia by:
iNova Pharmaceuticals (Australia) Pty Limited
ABN 13 617 871 539
Level 10, 12 Help Street
Chatswood NSW 2067
Tel: 1800 630 056
NORFLEX is distributed in New Zealand by:
Douglas Pharmaceuticals Ltd
Central Park Drive, Lincoln
AUCKLAND 0610.
This leaflet was prepared in November 2017
AUST R 10573
™ = Trademark
Published by MIMS February 2018

 Molecular Formula: C18H23NO.C6H8O7.
Molecular Formula: C18H23NO.C6H8O7.