What is in this leaflet
This leaflet answers some of the common questions about Ozidal (risperidone) tablets.
It does not contain all of the available information.
It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking this medicine against the benefits it is expected to have for you.
If you have any concerns about using Ozidal, ask your doctor or pharmacist. Your doctor and pharmacist have more information.
Keep this leaflet with your Ozidal tablets. You may need to read it again.
What Ozidal is used for
Ozidal is used to treat symptoms of schizophrenia and other types of related psychoses. These are disorders related to thought, feeling and/or action.
Ozidal may be taken for both sudden (acute) and long-lasting (chronic) schizophrenia.
Ozidal is also used for the short-term treatment of acute mania associated with bipolar 1 disorder. This condition is characterised by symptoms such as elevated, expansive or irritable mood, inflated self esteem, decreased need for sleep, pressured speech, racing thoughts, distractibility or poor judgement including disruptive or aggressive behaviours.
Ozidal is also used to treat behavioural problems in patients with a decline in mental abilitiy (dementia) caused by Alzheimer's disease. These problems include: aggression through words or action, morbid suspiciousness, agitation or wandering.
Ozidal can be used to treat conduct and other disruptive behaviours such as aggression, impulsiveness and self-injury in children (over 5 years old), adolescents and adults who are intellectually disabled.
Ozidal can also be used to treat behavioural symptoms of autism in children and adolescents.
Ozidal helps to correct a chemical imbalance in the brain associated with these conditions.
Ozidal has been approved for the uses mentioned above. However, your doctor may prescribe this medicine for another use. If you want more information, ask your doctor.
Ozidal is not addictive.
Before you take Ozidal
When you must not use it
Do not use Ozidal:
- If you know you are allergic to any of its ingredients (signs of allergy include skin rash, itching, shortness of breath, and/or swollen face - see the last section of this leaflet for a list of ingredients).
- If you are intolerant to lactose. This medicine contains small quantities of the inactive ingredient lactose. In case your doctor has told you that you have intolerance to some sugars, you must not take these tablets and should talk to your doctor. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption (which might cause pain in the abdomen, cramps, diarrhoea and gas after taking food) should not take these tablets.
- If the packaging is torn or shows signs of being tampered with.
- If the tablets do not look right.
- To treat any other complaints unless your doctor says it is safe to do so.
- After the expiry date printed on the pack. If you take it after the expiry date has passed, it may not work as well or may cause harm.
Before you start to use it
- Tell your doctor if:
- you have allergies to
- any other medicines
- any other substance such as foods, preservatives or dyes - you have any eye surgery planned
Your doctor will need to assess whether you are at risk of a surgical complication (called 'Intraoperative Floppy Iris Syndrome). You may be recommended to stop your Ozidal temporarily prior to your eye surgery. - Tell your doctor if you are pregnant or planning to become pregnant.
Your doctor will advise you whether or not you should take Ozidal. Agitation, muscle stiffness, unusual muscle slackness, shaking, drowsiness, breathing disorder and difficulty in feeding may occur in newborns, if a mother used risperidone in the last trimester of her pregnancy. - Tell your doctor if you are breast-feeding or planning to breastfeed.
Ozidal is excreted in breast milk. It is recommended that you do not breast-feed while taking Ozidal. - you will be in a hot environment or do a lot of vigorous exercise.
Ozidal may make you sweat less, causing your body to overheat.
- Tell your doctor if you have or have ever had any medical conditions, especially the following:
- Heart or blood vessel diseases, including low blood pressure.
Low blood pressure can result from using risperidone together with medicines to treat high blood pressure. So, if you need to use both risperidone and medications to reduce blood pressure, consult your doctor
Risperidone should be used with caution, and only after consultation with your doctor, if you have heart problems, particularly irregular heart rhythm, abnormalities in electrical activity of the heart, or if using medications that can change the heart’s electrical activity. - unusual excessive sweating or diarrhea, dehydration or problems with your body temperature regulation
- kidney or liver problems
- you are prone to dizziness when standing up from lying or sitting position
- Parkinson's disease (a progressive movement and thinking disorder that tends to affect older people)
- dementia or Lewy body dementia. Older people suffering from dementia may be at increased risk of stroke or death with Ozidal
- epilepsy, seizures or fits
- continuous and/or painful erections (called 'priapism')
- low potassium levels (hypokalaemia)
- breast cancer
- cancer of the pituitary gland
- sugar diabetes
- unusual thirst, tiredness, blurred vision, upset stomach or need to urinate - common signs of high blood sugars
- disease of the blood vessels of the brain including stroke
- tardive dyskinesia (a reaction to some medicines with uncontrollable twitching or jerking movements of the tongue, face, mouth, jaws, arms and legs)
- Neuroleptic Malignant Syndrome (a serious reaction to some medicines with a sudden increase in body temperature, very fast heartbeat, extremely high or low blood pressure and severe convulsions)
- blood clots
Tell your doctor if you or someone else in your family has a history of blood clots. Blood clots in the lungs and legs can occur with Ozidal. Blood clots in the lungs can result in death. - Involuntary movements or unusual restlessness or difficulty sitting still (akathisia)
- suicidal thoughts or past suicide attempts
- low white blood cell count
- If you have low numbers of some white blood cells, your risk of contracting an infection or developing a fever is increased with Ozidal.
If you have not told your doctor about any of the above, tell him / her before you start taking Ozidal.
Avoid alcoholic beverages until you have discussed their use with your doctor. Ozidal can increase the effect of alcohol. You should not drink alcohol while taking Ozidal.
Taking other medicines
Tell your doctor if you are taking any other medicines, including medicines you buy without a prescription from pharmacy, supermarket or health food shop.
Some medicines may affect the way other medicines work.
In particular tell your doctor if you are taking:
- sleeping tablets, tranquillisers, pain-killers, certain allergy medicines called antihistamines, certain antidepressants
- medicines that increase the activity of the central nervous system (psychostimulants such as methylphenidate)
- medicines used to treat bacterial infections such as rifampicin
- medicines to treat fungal infections such as itraconazole and ketoconazole
- medicines to treat HIV/AIDS, such as ritonavir and tipranavir
- carbamazepine, a drug mainly used for epilepsy or trigeminal neuralgia (severe pain attacks in the face), as it may decrease the level of risperidone in your blood
- medicines to treat Parkinson's disease or tremor
- medicines to treat epilepsy
- medicines to treat depression, panic disorder, anxiety or obsessive-compulsive disorder. For example fluoxetine and paroxetine may increase the level of risperidone in your blood. So tell your doctor if you start and/or stop taking fluoxetine or paroxetine.
- diuretics
- medicines for your heart or blood pressure
- verapamil, a medicine used to treat high blood pressure and/or abnormal heart rhythm
- frusemide, a medicine used to treat high blood pressure and fluid build-up.
There is an increased risk of side effects or death in older people if frusemide is also taken with Ozidal. - other medicines to treat mental illness or psychotic conditions
Your doctor or pharmacist has more information on medicines to be careful with or avoid while taking Ozidal.
Elderly People
Elderly people should take less Ozidal than is prescribed for other adults (see “How to take it”).
Using Ozidal
Taking it for the first time
At the start of treatment you may have a fall in blood pressure making you feel dizzy on standing up, or your heart may beat faster. These should go away after a few days. Tell your doctor if they continue or worry you.
How much to take
Take only when prescribed by your doctor.
Ozidal may be taken as a single dose, once a day or it may be taken in divided doses twice a day (in the morning and in the evening).
It is very important that you take the correct amount of Ozidal, but this will vary from person to person. Your doctor will adjust the number and strength of the tablets until the desired effect is obtained.
Follow your doctor's instructions carefully and do not change or stop the required dosage without consulting your doctor first.
For Schizophrenia and Related Psychoses
The usual starting dose of Ozidal is 1 mg twice a day. This will be gradually increased by your doctor to suit your needs.
From then on, the dose can be taken once a day or twice a day according to your doctor's instructions. For long-term treatment, 4 to 6 milligrams per day is usually sufficient but your doctor will determine the dose most suitable for you.
Important note: never take more tablets than your doctor tells you to take.
The effects of high doses are not yet known. Please double check with your doctor if your doctor prescribes more than 5 milligrams twice a day.
Ozidal cannot be recommended for use in children with schizophrenia under 15 years at the present time as there is little experience with the product in this group.
For Elderly Patients with Schizophrenia or Related Psychoses
For older patients a starting dose of 0.5 mg (half a 1 mg tablet) twice a day (in the morning and in the evening) is usual. The dose may be increased by 0.5 mg twice daily to 1 to 2 mg twice a day (in the morning and in the evening).
Patients with impaired kidney and liver function
If you have kidney or liver disease a starting dose of 0.5 mg (half a 1 mg tablet) twice a day (in the morning and in the evening) is usual. The dose may be increased by 0.5 mg twice daily to 1 to 2 mg twice a day (in the morning and in the evening).
For acute mania
The recommended starting dose is 2 mg once a day. This dose can be adjusted by dose increases of 1mg when needed every 24 hours. Most people feel better with doses between 2 mg and 6 mg a day. Your doctor may decide you should take another drug called a mood stabiliser as well as Ozidal.
For Behavioural Problems in People with Dementia
The usual starting dose is 0.25 mg twice daily. This may be gradually increased by your doctor to suit your needs.
From then on the dose can be taken once a day or twice a day according to your doctor’s instructions. For long-term treatment, 1 mg daily is the usual dose but your doctor will determine the most suitable dose for you.
For Disruptive Behaviour Disorders in Adults and Children
For people who weigh 50 kg or more, the usual starting dose is 0.5 mg (half a 1 mg tablet) once a day. The dose may be increased by 0.5 mg once every two days, to the usual dose of 0.5 to 1.5 mg once a day.
For people who weigh less than 50 kg, the usual starting dose is 0.25 mg once a day. The dose may be increased by 0.25 mg once every two days, to the usual dose of 0.25 to 0.75 mg once a day.
Your doctor will advise you on how much Ozidal you need.
Ozidal cannot be recommended for use in children with disruptive behaviour disorders under 5 years at the present time as there is little experience with the product in this group.
For Behavioural Disorders Associated with Autism in Children and Adolescents
For people weighing 20 kg or less the usual starting dose is 0.25 mg. On day 4 this dose can be increased to 0.5 mg.
For people weighing more than 20 kg the usual starting dose is 0.5 mg. On day 4 this dose can be increased to 1 mg.
Response should be assessed at day 14; only in patients not achieving sufficient clinical response should additional dose increases be considered. Your doctor will advise you on how much Ozidal you need. When trialled, the maximum dose of risperidone in patients with autism did not exceed 1.5 mg/day in patients less than 20 kg, 2.5 mg in patients weighing 20kg or more, or 3.5mg in patients weighing more than 45 kg.
Follow all directions given to you by your doctor carefully.
They may differ from the information contained in this leaflet.
If you do not understand the instructions on the box, ask your doctor or pharmacist for help.
When and how to take it
Ozidal tablets should be swallowed with water or other liquid.
You may take Ozidal either with or between meals.
How long to take it
Continue taking your medicine for as long as your doctor tells you.
Do not let yourself run out of medicine over the weekend or on holidays.
If you forget to take it
If you forget to take Ozidal, take the missed dose as soon as you remember instead of your next dose. Then go back to taking it as you would normally.
Do not take a double dose to make up for the one you missed. If you forget to take Ozidal for 5 days or more, tell your doctor before starting your medicine again.
If you have problems remembering when to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much Ozidal. Do this even if there are no signs of discomfort or poisoning.
You may need urgent medical attention. Please keep these phone numbers handy.
Signs of overdose may include drowsiness, sleepiness, excessive trembling, excessive muscle stiffness, increased heart rate, very low blood pressure causing fainting or unconsciousness.
While you are using Ozidal
Things you must do
Always follow your doctor's instructions carefully, and seek your doctor's advice before changing or stopping treatment. Your doctor will be happy to discuss any questions you may have with your treatment.
Contact your doctor if you notice any involuntary movements of the tongue, mouth, cheeks or jaws, which may progress to arms and legs.
These may be symptoms of a condition called Tardive Dyskinesia, which can develop in people taking antipsychotic medicines, including Ozidal. This condition is more likely to occur during longer term treatment and in older women. In very rare cases, these symptoms may be permanent. However, if detected early, these symptoms are usually reversible.
Be careful during strenuous exercise or exposure to extreme heat. Try to drink plenty of water.
Visit your doctor regularly for check ups.
If you suffer from sugar diabetes your doctor may monitor your blood sugar levels regularly to check for worsening of glucose control.
Pre-menopausal women should tell their doctor if they do not have a period for more than six months while taking Ozidal, even if you are not pregnant.
Tell any other doctors, dentists, and pharmacists who treat you that you are taking this medicine.
Try to eat a moderate diet. Ozidal can cause weight gain.
Things you must not do
- Do not take Ozidal to treat any other complaints unless your doctor tells you to.
- Do not give your medicine to anyone else, even if they have the same condition as you.
- Do not stop taking your medicine or lower the dosage without checking with your doctor.
- Do not drink alcohol. Ozidal can increase the effects of alcohol.
Things to be careful of
- Ask your doctor before taking any other medicines. Ozidal can increase the effects of medicines which slow your reactions. Always ask your doctor or pharmacist before taking other medicines. These include herbal treatments and those bought in a pharmacy or supermarket.
- Avoid driving or operating machinery until you know how Ozidal affects you. Ozidal may cause dizziness or light-headedness in some people, especially after the first dose. Make sure you know how you react to Ozidal before you drive a car, operate machinery, or do anything else that could be dangerous if you are dizzy.
- Avoid excessive eating, as there is a possibility of weight gain when taking Ozidal.
- If the medicine makes you feel light-headed, dizzy or faint, be careful when getting up from a sitting or lying position.
- Getting up slowly may help.
- There is a possibility of weight gain when taking Ozidal. Your doctor may monitor your body weight or recommend strategies to assist with weight management.
Side Effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Ozidal.
Ozidal helps most people but it may have some unwanted side effects in a few people. All medicines can have side effects. Sometimes they are serious, but most of the time they are not. Risperidone is generally well-tolerated and side effects are often hard to distinguish from the disease symptoms. You may need medical treatment if you get some of the side effects.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking RISPERDAL
Tell your doctor if you notice any of the following and they worry you:
- difficulty thinking, working or carrying out your usual daily activities because of:
- headache
- trembling, muscle weakness, unsteadiness on your feet, lack of coordination or slow, shuffling walk (symptoms of Parkinsonism).
- lack of energy, drowsiness or excessive sleeping during the day, sleeplessness or difficulty in concentrating
- difficulty speaking
- blurred vision
- fainting
- dizziness
- any problems with confusion or unsteadiness
- pains in parts of your body, e.g. in the neck, back, ear, hands or feet
- failing - muscle, joint, nerve or movement changes such as:
- shaking or trembling
- fatigue or weakness
- muscle stiffness
- restlessness in the legs or difficulty sitting still
- uncontrolled muscle spasms, twitching, jerky or writhing movements
- muscle aches or pain
- joint swelling or pain
- walking abnormally or with difficulty
- abnormal posture, such as rigid body movements and persistent abnormal positions of the body - behavioural changes such as:
- irritability or agitation
- unusual anxiety or nervousness - other changes such as:
- cold or "flu-like" symptoms e.g. cough, blocked or runny nose, sneezing, sore throat
- feeling of tension or fullness in the nose, cheeks and behind your eyes, sometimes with a throbbing ache, fever, stuffy nose and loss of the sense of smell (signs of sinusitis)
- tiredness, headaches, being short of breath when exercising, dizziness and
- looking pale (signs of decreased red blood cells)
- fever, chills, shortness of breath, cough, phlegm and occasionally blood (signs of pneumonia)
- nosebleeds
- discharge with itching of the eyes and crusty eyelids
- unexplained weight gain
- unexplained increase or decrease in appetite
- indigestion, stomach discomfort or pain, diarrhoea or constipation
- nausea or vomiting
- dry mouth or excessive thirst
- drooling
- difficulty swallowing
- acne
- dry skin
- rash, red skin or itchy skin
- thickening of the skin resulting in warts, corns, calluses
- skin infection
- swelling of any part of your body, e.g. hands, ankles or feet
- inability to or feeling burning pain when passing urine
- some loss of bladder control
- bedwetting
- frequent daytime urination in children
- sexual function disturbances - problems with ejaculation
- breast abnormalities - breast discomfort or swelling or unusual secretion of breast milk
- missed or irregular menstrual periods
- dizziness on standing up, especially when getting up from a sitting or lying down position
- shortness of breath
- chest pain or discomfort
- an increase of CPK (creatine phosphokinase) in your blood, an enzyme which is sometimes released with muscle breakdown.
These can only be detected by blood tests that your doctor may ask to be done.
These are mild side effects of Ozidal but may require medical attention.
Tell your doctor immediately, or go to Accident and Emergency at your nearest hospital if you notice any of the following:
- Signs of heart or blood pressure problems including:
- fainting, blurry vision, light-headedness or dizziness particularly on standing that persists despite sitting or lying down again
- very fast heart rate, slowed heart rate, heart rhythm irregularities - Signs of lung problems including:
- sudden shortness of breath, trouble breathing, wheezing or gasping when you breathe, light-headedness or dizziness - signs of high blood sugar or diabetes such as:
- unusual thirst, tiredness, upset stomach or need to urinate more often than usual - body temperature changes such as:
- fever
- unexplained high body temperature, excessive sweating or rapid breathing
- severe muscle stiffness or fits - involuntary movements of the tongue, face, mouth, jaw, arms, legs or trunk
- rash, itching or hives on the skin; shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue or other parts of the body.
If you have them, you may have had a serious allergic reaction to OzidaL. - sudden weakness or numbness of the face, arms, or legs, especially on one side, or instances of slurred speech (these are called ministrokes)
These are very serious side effects. You may need urgent medical attention or hospitalisation.
Tell your doctor if you notice anything else that is making you feel unwell. Other side effects not listed above may occur in some patients.
Do not hesitate to report any other side effects to your doctor or pharmacist.
Do not be alarmed by this list of possible side effects.
You may not experience any of them.
After using it
Storage
Keep Ozidal tablets in a dry place where the temperature stays below 25°C.
Do not store it or any medicines in the bathroom or near a sink. Heat and dampness can destroy some medicines.
Keep your tablets in the pack until it is time to take them. If you take your tablets out of the pack they will not keep as well.
Keep it where young children cannot reach it. A locked cupboard at least one-and-a-half meters above the ground is a good place to store medicines.
Do not use Ozidal tablets beyond the date (month and year) printed on the pack after the letters EXP, even if it has been stored properly. Medicines cannot be stored indefinitely.
Do not use Ozidal if the appearance of the tablets has changed.
Disposal
Once you have finished using Ozidal, ask your pharmacist what to do with any unused medicine.
Product Description
What it looks like
Ozidal 0.5 mg tablets are brown-coloured, film-coated, oval-shaped tablets with a scoreline on both sides and ‘RSN’ and ‘0.5’ debossed on either side of the scoreline on one side, available in packs of 60 tablets.
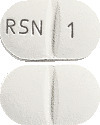
Ozidal 1 mg tablets are white, film-coated, capsule-shaped tablets with a scoreline on both sides and ‘RSN’ and ‘1’ debossed on either side of the scoreline on one side, available in packs of 60 tablets.
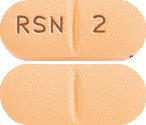
Ozidal 2 mg tablets are peach-coloured, film-coated, capsule-shaped tablets with a scoreline on both sides and ‘RSN’ and ‘2’ debossed on either side of the scoreline on one side, available in pack of 60 tablets.
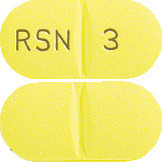
Ozidal 3 mg tablets are yellow-coloured, film-coated, capsule-shaped tablets with a scoreline on both sides and ‘RSN’ and ‘3’ debossed on either side of the scroreline on one side, available in pack of 60 tablets.
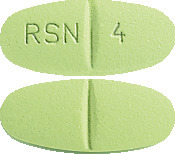
Ozidal 4 mg tablets are green-coloured, film-coated, capsule-shaped tablets with a scoreline on both sides and ‘RSN’ and ‘4’ debossed on either side of the scoreline on one side, available in pack of 60 tablets.
Ingredients
Active ingredient
Ozidal 0.5 mg tablets - 0.5 mg of risperidone
Ozidal 1 mg tablets - 1 mg of risperidone
Ozidal 2 mg tablets - 2 mg of risperidone
Ozidal 3 mg tablets - 3 mg of risperidone
Ozidal 4 mg tablets - 4 mg of risperidone
Inactive ingredients
Lactose monohydrate, pregelatinised starch, microcrystalline cellulose, sodium lauryl sulphate, colloidal anhydrous silica and magnesium stearate. In addition the 0.5 mg tablet contains Opadry II 31G 56729 brown, 1 mg tablet contains Opadry 20H 58983 white, the 2 mg tablet contains Opadry II 31G 53291 orange; the 3 mg tablet contains Opadry II 31G 52408 yellow and the 4 mg tablet contains Opadry II 31G 51195 green.
Australian Registration Numbers
Ozidal 0.5 mg tablets - AUST R 148964
Ozidal 1 mg tablets - AUST R 148967
Ozidal 2 mg tablets - AUST R 148968
Ozidal 3 mg tablets - AUST R 148969
Ozidal 4 mg tablets - AUST R 148970
Sponsor
Sun Pharma ANZ Pty Ltd.
Macquarie Park, NSW 2113
Australia
[email protected]
Tel: 1800 726 229
This leaflet was prepared in October 2019
Published by MIMS August 2020


 For prescribers preferring to dose on a mg/kg/day basis the following guidance is provided in Table 2.
For prescribers preferring to dose on a mg/kg/day basis the following guidance is provided in Table 2. Patients experiencing somnolence may benefit from a switch in dosing from once daily to either once daily at bedtime or twice daily.
Patients experiencing somnolence may benefit from a switch in dosing from once daily to either once daily at bedtime or twice daily.


 ADRs reported with risperidone and/or paliperidone by < 1% of risperidone treated subjects in a pooled dataset of 23 double-blind, placebo-controlled pivotal studies (9 in adult, 8 in paediatric patients, 6 in elderly patients with dementia) are shown in Table 7.
ADRs reported with risperidone and/or paliperidone by < 1% of risperidone treated subjects in a pooled dataset of 23 double-blind, placebo-controlled pivotal studies (9 in adult, 8 in paediatric patients, 6 in elderly patients with dementia) are shown in Table 7. ADRs reported with risperidone and/or paliperidone in other clinical trials but not reported by risperidone-treated subjects in a pooled dataset of 23 double-blind, placebo-controlled pivotal studies are shown in Table 8.
ADRs reported with risperidone and/or paliperidone in other clinical trials but not reported by risperidone-treated subjects in a pooled dataset of 23 double-blind, placebo-controlled pivotal studies are shown in Table 8.

 The rate of discontinuation from the pooled studies was similar for patients receiving placebo (30.2%), risperidone (33.5%) and haloperidol (29.6%). In the combined analysis, risperidone, at a daily dose above 0.75 mg, effectively reduces the severity (measured by means of the Behave-AD) and frequency (measured by the CMAI) of aggressiveness symptoms in this patient population. Reductions in Behave-AD aggressiveness scores and on each of the aggressive clusters of the CMAI were significantly greater with risperidone (doses above 0.75 mg/day) than placebo at endpoint in both studies and in the combined analysis. Reductions in CMAI total aggressive scores declined throughout the studies in the risperidone patients but changed minimally after Week 2 in patients receiving haloperidol or placebo.
The rate of discontinuation from the pooled studies was similar for patients receiving placebo (30.2%), risperidone (33.5%) and haloperidol (29.6%). In the combined analysis, risperidone, at a daily dose above 0.75 mg, effectively reduces the severity (measured by means of the Behave-AD) and frequency (measured by the CMAI) of aggressiveness symptoms in this patient population. Reductions in Behave-AD aggressiveness scores and on each of the aggressive clusters of the CMAI were significantly greater with risperidone (doses above 0.75 mg/day) than placebo at endpoint in both studies and in the combined analysis. Reductions in CMAI total aggressive scores declined throughout the studies in the risperidone patients but changed minimally after Week 2 in patients receiving haloperidol or placebo. Following completion of Study 1, 63 patients entered an open-label extension for up to 4 additional months. Thirty nine patients who were clinically stable and who showed a positive response to risperidone after 6 months were then randomised to receive risperidone or placebo during an 8-week, double blind withdrawal period. The relapse rate was 11/16 and 2/16 in placebo and risperidone treated patients respectively (Odds Ratio 15.4, 95% confidence limits 2.50, 95.05).
Following completion of Study 1, 63 patients entered an open-label extension for up to 4 additional months. Thirty nine patients who were clinically stable and who showed a positive response to risperidone after 6 months were then randomised to receive risperidone or placebo during an 8-week, double blind withdrawal period. The relapse rate was 11/16 and 2/16 in placebo and risperidone treated patients respectively (Odds Ratio 15.4, 95% confidence limits 2.50, 95.05). As few autistic children with an IQ > 84 are seen, there is limited clinical experience with risperidone in such children. Experience in autistic adolescents is also limited.
As few autistic children with an IQ > 84 are seen, there is limited clinical experience with risperidone in such children. Experience in autistic adolescents is also limited.
