What is in this leaflet
This leaflet answers some common questions about OZVIR (aciclovir).
It does not contain all the available information.
It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking this medicine against the benefits it is expected to have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with this medicine. You may need to read it again.
What OZVIR is used for
OZVIR contains aciclovir, a compound that belongs to a group of medicines called antivirals.
OZVIR is used for the treatment of shingles (herpes zoster), impaired immunity and genital herpes.
In the case of shingles, OZVIR works by stopping the multiplication of the virus which causes shingles. It can reduce the length and severity of an outbreak of shingles but it will not get rid of the virus from your body.
Aciclovir at high strength is used as part of the management program in people who have human immunodeficiency virus disease (HIV). It acts by preventing further damage to the immune system. In these people, OZVIR also guards against the herpes virus disease.
Aciclovir does not get rid of the virus from your body.
Your doctor may have prescribed OZVIR for another purpose.
OZVIR is not recommended for use in children as there have been no studies of its effects in children.
Ask your doctor if you have any questions about why OZVIR has been prescribed for you.
This medicine is only available with a doctor's prescription.
Before you take OZVIR
When you must not take it
Do not take OZVIR if:
- you have ever had an allergic reaction to OZVIR or to any of the ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include a skin rash similar to hives, itchiness, shortness of breath, swelling of the face, lips or tongue.
If you are not sure whether you should be taking OZVIR, talk to your doctor.
Do not take OZVIR if:
- the packaging shows signs of tampering or does not look quite right.
- the expiry date marked on the packaging has passed, even though the tablets may look alright. If it has expired or is damaged, return it to your pharmacist for disposal.
Before you start to take it
Tell your doctor if:
- you are pregnant or intend to become pregnant while taking OZVIR.
Medicines like OZVIR may affect the developing baby if you take it during pregnancy. Your doctor will discuss the possible risks and benefits of taking OZVIR during pregnancy. - you are breast-feeding or wish to breast-feed.
Your doctor will discuss the risks and benefits of taking OZVIR when breast-feeding. - you are allergic to any foods, dyes, preservatives or any other medicines.
- you have any health problems, including:
- kidney or liver problems
- neurological problems
- receiving interferon or methotrexate treatment
- insufficient oxygen level in blood or tissue, or altitude sickness
Symptoms are dizziness, shortness of breath, and mental confusion
Taking other medicines
Tell your doctor if you are taking any other medicines, including medicines you buy without a prescription from a pharmacy, supermarket or health food shop. Some medicines and OZVIR may interfere with each other.
Your doctor or pharmacist has a complete list of medicines to be careful with or avoid while taking OZVIR.
If you have not told your doctor or pharmacist about these things, tell them before you start taking OZVIR.
How OZVIR is given
How to take it
Take OZVIR exactly as your doctor has prescribed.
Follow all directions given to you by your doctor or pharmacist. They may differ from the information contained in this leaflet.
How much to take
Treatment for Shingles
The normal dose is to take 800 mg five times a day approximately every 4 hours throughout the day while awake, for 7 days. Either take your tablets at 7am, 11am, 3 pm, 7pm and 11pm or note down the times that suit your daily schedule.
Management of advanced HIV
The dose is 800 mg four times a day every 6 hours, for as long as your doctor tells you.
Treatment of initial genital herpes
One 200 mg tablet every four hours while awake, for a total of 5 tablets daily, for 10 days.
Intermittent therapy for recurrent genital herpes
One 200 mg tablet every four hours while awake, for a total of 5 tablets daily, for 5 days. Therapy should be initiated at the earliest sign or symptom (prodrome) of recurrence.
Chronic suppressive therapy for recurrent genital herpes
One 200 mg tablet three times a day for up to 6 months. For most people, one 200 mg tablet twice a day gives satisfactory results.
The Pharmacist’s label on the pack will give the dosage instructions for your treatment.
OZVIR tablets may be dispersed in a glass of water prior to being taken or alternatively swallow the tablets whole with a glass of water. You should drink plenty of fluids.
If you do not understand the instructions on the box ask your doctor or pharmacist for help.
How long to take it
You must take OZVIR for as long as your doctor tells you. Do not stop taking OZVIR just because you feel better.
If you forget to take it
If you forget to take a dose, take one as soon as you remember, unless this is within an hour of when the next dose is due. Then go on as before.
Do not take a double dose to make up for the dose that you missed. This may increase the chance of getting an unwanted side effect.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to accident and emergency (Casualty) at your nearest hospital if you think you or anyone else may have taken too much OZVIR. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention. Keep telephone numbers of these places/services handy.
While you are using OZVIR
Things you must do
Take OZVIR tablets exactly as your doctor tells you to.
Visit your doctor regularly for check-ups.
Tell any other doctors, dentists and pharmacists who are treating you that you are taking OZVIR
If you are about to start any new medicine, tell your doctor or pharmacist that you are taking OZVIR
If you become pregnant while taking OZVIR tell your doctor immediately.
Things you must not do
Do not take OZVIR to treat any other conditions unless your doctor says to.
Do not give this medicine to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or lower the dosage without checking with your doctor.
Things to be careful of
Be careful driving or operating machinery until you know how OZVIR affects you. OZVIR may cause dizziness or drowsiness in some people and affect alertness.
If this occurs, do not drive, operate machinery or do things that could be dangerous if you are not alert.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking OZVIR. OZVIR helps most people but it may have some unwanted side effects in a few people. All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Do not be alarmed by the list of possible side effects. You may not experience any of them.
Tell your doctor if you experience any of the following and they worry you:
- Nausea
- Vomiting
- Headache
These are the more common side effects of OZVIR.
Tell your doctor if you experience any of the following:
- Dizziness, fatigue, fast heart rate, fever, chills, short of breath
- Confusion, sleep disturbances, hallucinations, shakiness, irritability
- Weight loss, fluid retention, pain in the leg and joints, vein disorders
- Diarrhoea, constipation
- Sore throat, taste disturbances, swollen glands
- Skin rashes, hair loss
- Cramps, menstrual problems
- Disorders of the eyes and liver
These may or may not be due to OZVIR but you should tell your doctor if they worry you.
This is not a complete list of all possible side effects. Others may occur in some people and there may be some side effects not yet known.
If you notice any other symptoms that worry you, check with your doctor.
Ask your doctor or pharmacist if you don't understand anything in this list.
After using it
Storage
Keep OZVIR where children cannot reach it.
Keep your tablets in the pack until it is time to take them. If you take your tablets out of the pack they may not keep as well.
Keep your tablets in a cool dry place where temperatures stay below 25°C. Protect from light.
Do not store OZVIR or any other medicine in the bathroom or near a sink.
Do not leave your medicines on a window sill or in the car. Heat and dampness can destroy some medicines.
Disposal
If your doctor tells you to stop taking OZVIR, or the tablets have passed their expiry date, ask your pharmacist what to do with any left over.
Product description
What it looks like
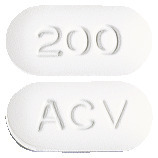
OZVIR tablets are capsule-shaped, biconvex, uncoated, white to off-white tablets with 200 embossed on one side and ACV on the other side. Available in blister packs of 90 tablets.
Ingredients
Active ingredient:
Aciclovir
Inactive ingredients:
Microcrystalline cellulose, sodium starch glycollate, starch-pregelatinised maize, silica-colloidal anhydrous and magnesium stearate.
Australian Registration Numbers
OZVIR 200 mg tablets blister pack: AUST R 117380
Sponsor
OZVIR tablets are supplied in Australia by:
Ranbaxy Australia Pty. Ltd
Suite 4.02, Building D, Level 4
12-24 Talavera Road
North Ryde, NSW 2113
Australia
This leaflet was prepared in Mar 2011.
Published by MIMS January 2012