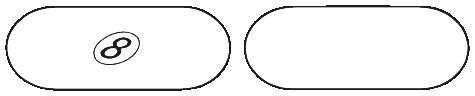What is in this leaflet
This leaflet answers some common questions about PANADOL OSTEO. It does not contain all the available information.
It does not take the place of talking to your pharmacist or doctor.
All medicines have risks and benefits.
If you have any concerns about using this medicine, ask your pharmacist or doctor.
Keep this leaflet with the medicine. You may need to read it again.
What PANADOL OSTEO is used for
PANADOL OSTEO is effective for the relief of persistent pain associated with:
- Osteoarthritis
- Muscle aches and pains such as backache
Paracetamol, the active ingredient in this medicine, is also used for the fast effective temporary relief of pain and discomfort associated with:
- Headache
- Tension headache
- Period pain
- Toothache and pain after dental procedures
- Colds and flu
Paracetamol also reduces fever.
Paracetamol works to stop the pain messages from getting through to the brain. It also acts in the brain to reduce fever.
Ask your pharmacist or doctor if you have any questions about this medicine. Your pharmacist or doctor may have recommended it for another reason.
Before you use PANADOL OSTEO
Do not take more than the recommended dose as it may cause serious harm to your liver.
When you must not use it
Do not use PANADOL OSTEO if you have an allergy to:
- Any medicine containing paracetamol
- Any of the ingredients listed at the end of this leaflet
Do not take this medicine if you are taking other prescription or non-prescription medicines containing paracetamol to treat pain, fever, symptoms of cold and flu, or to aid sleep.
Always read and follow the label.
Do not use this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start using this medicine, talk to your pharmacist or doctor.
Before you start to use it
Tell your pharmacist or doctor if you have or have had any of the following medical conditions:
- Liver or kidney disease
- Are underweight or malnourished
- Regularly drink alcohol
You may need to avoid using this product altogether or limit the amount of paracetamol that you take. - You have a severe infection, are severely malnourished or are a chronic heavy alcohol user as this may increase the risk of metabolic acidosis:
Signs of metabolic acidosis include:
- deep, rapid, difficult breathing
- feeling sick (nausea), being sick (vomiting)
- loss of appetite
Contact a doctor immediately if you get a combination of these symptoms.
Please see your doctor if your symptoms do not improve.
Keep out of sight and reach of children.
Ask your pharmacist or doctor about using paracetamol if you are pregnant or plan to become pregnant, or breastfeeding. Paracetamol may be used during pregnancy and if you are breastfeeding but you should always consult your doctor first.
Consider taking the lowest effective dose for the shortest period of time.
If you have not told your pharmacist or doctor about any of the above, tell them before you use PANADOL OSTEO.
Using other medicines
Tell your pharmacist or doctor if you are using any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and paracetamol may interfere with each other. These include:
- Warfarin, a medicine used to prevent blood clots
- Metoclopramide, a medicine used to control nausea and vomiting
- Medicines used to treat epilepsy or fits
- Chloramphenicol, an antibiotic used to treat ear and eye infections
- Alcohol
- Probenecid, a medicine used to treat gout or sometimes given with an antibiotic
- Cholestyramine, a medicine used to treat high cholesterol levels in the blood.
Your pharmacist and doctor will have more information on these and other medicines to be careful with or avoid while using this medicine.
How to use PANADOL OSTEO
Follow all directions given to you by your pharmacist or doctor carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the box, ask your pharmacist or doctor for help.
Do not exceed the stated dose.
Use the smallest dose that you need to treat your symptoms and use the medicine for the shortest period of time necessary.
How much to use
Adults and children aged 12 years and over:
Take 2 caplets three times a day, every six to eight hours as needed. Do not take more than 6 caplets in 24 hours.
Not recommended in children under 12 years.
How to use it
Swallow the caplets whole with water or other fluid.
Do not crush the caplets. They can be taken with or without food.
Try to space the doses at equal intervals throughout the day.
How long to use it
Adults and children aged 12 years and over:
Only take paracetamol for a few days at a time unless your doctor tells you to take it for longer.
If you use too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26 for Australia. 0800 764 766 for New Zealand) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much PANADOL OSTEO. Do this even if there are no signs of discomfort or poisoning because of the risk of liver failure. You may need urgent medical attention.
While you are using PANADOL OSTEO
Things you must do
Talk to your pharmacist or doctor if your symptoms do not improve. Your pharmacist or doctor will assess your condition and decide if you should continue to take the medicine.
Things you must not do
Do not use for more than a few days at a time unless your doctor tells you to.
Do not take more than the recommended dose unless your doctor tells you to.
Do not use PANADOL OSTEO to treat any other complaints unless your pharmacist or doctor tells you to.
Things to be careful of
Only drink small quantities of alcohol (beer, wine or spirits) while using PANADOL OSTEO. Drinking large quantities of alcohol whilst taking PANADOL OSTEO may increase the risk of liver side effects.
Side Effects
Tell your pharmacist or doctor as soon as possible if you do not feel well while you are using PANADOL OSTEO.
This medicine helps most people with various types of pain but it may have unwanted side effects. All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by the following list of side effects. These side effects are rare and you may not experience any of them.
Ask your pharmacist or doctor to answer any questions you may have.
If any of the following happen, stop using the product and tell your pharmacist or doctor immediately or go to Accident and Emergency at your nearest hospital:
- Shortness of breath
- Wheezing or difficulty breathing
- Swelling of the face, lips, tongue or other parts of the body
- Rash, peeling, itching or hives on the skin or mouth ulcers
- Unexplained bruising or bleeding
The above list includes very serious side effects. You may need urgent medical attention or hospitalisation. These side effects are very rare for low doses of this medicine and when used for a short period of time.
Tell your pharmacist or doctor if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some people.
After using PANADOL OSTEO
Storage
Keep your medicine in the original pack until it is time to take it.
Keep your medicine in a cool dry place where the temperature stays below 30°C.
Do not store PANADOL OSTEO or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in a car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
Ask your pharmacist what to do with any medicine that is left over, or if the expiry date has passed.
Product Description
What it looks like
PANADOL OSTEO caplets are a white, to off-white, film-coated capsule-shaped tablet with flat edges. They are marked "8" on one side and are plain on the other side. They come in blister packs of 6,12 and 96 caplets.
Ingredients
PANADOL OSTEO caplets contain 665 mg of paracetamol as the active ingredient.
They also contain:
- Hypromellose
- Starch-pregelatinised maize
- Povidone
- Croscarmellose sodium
- Magnesium stearate
- Stearic acid
- Glycerol triacetate
- Carnauba wax
Manufacturer/Supplier
PANADOL OSTEO caplets is supplied in Australia and New Zealand by:
GlaxoSmithKline Consumer Healthcare,
82 Hughes Avenue, Ermington NSW and Auckland, New Zealand
AUST R 260264
Date of preparation: December 2019
Trademarks are owned by or licensed to the GSK group of companies
Published by MIMS October 2020