What is in this leaflet
This leaflet answers some common questions about PHARMACOR TACROLIMUS Capsules. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking PHARMACOR TACROLIMUS against the benefits this medicine is expected to have for you.
If you have any concerns about using PHARMACOR TACROLIMUS ask your doctor or pharmacist. Keep this leaflet with your medicine. You may need to read it again.
What PHARMACOR TACROLIMUS is used for
You have been given a new transplanted liver or kidney, lung or heart from another person because your own was no longer healthy. Your body recognises that this new organ is different from your other organs and will try to reject it by attacking it in the same way that it would attack germs that enter your body. This could make you become ill again. PHARMACOR TACROLIMUS stops this attack; it is very important to take PHARMACOR TACROLIMUS given to you by your doctor regularly so that your new liver, kidney, lung or heart will not be attacked or rejected.
If you have been taking other medicines for this purpose, but are still feeling unwell, your doctor may change your treatment and begin giving you PHARMACOR TACROLIMUS. PHARMACOR TACROLIMUS contains the active ingredient tacrolimus, which is an immunosuppressive agent.
Your doctor may have prescribed PHARMACOR TACROLIMUS for another reason.
Ask your doctor if you have any questions about why this medicine has been prescribed for you.
Before you use PHARMACOR TACROLIMUS
When you must not use it
Do not use PHARMACOR TACROLIMUS if you have an allergy to:
- Any medicine containing tacrolimus or other macrolides (these are antibiotics of the erythromycin family – trade names are Eryc, EES, Klacid, Zithromax, Rulide and Biaxsig)
- any of the ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin
Do not use PHARMACOR TACROLIMUS if the packaging is torn or shows signs of tampering. Do not use PHARMACOR TACROLIMUS beyond the expiry date printed on the pack.
Before you start to use it
You must tell your doctor if
- you are pregnant or planning to become pregnant
- you are using oral contraceptives
- you are breast feeding
- you are receiving cyclosporin immunosuppressive therapy.
If you have not told your doctor or pharmacist about any of the above, tell them before you start taking or are given PHARMACOR TACROLIMUS.
Your doctor will advise you whether or not to take PHARMACOR TACROLIMUS or if you need to adjust the dose or adapt your treatment.
Taking other medicines:
Tell your doctor or pharmacist if you are taking any other medicines, including medicines you can buy without a prescription from a pharmacy, supermarket or health food shop.
Some medicines and PHARMACOR TACROLIMUS may interfere with each other.
Among these medicines is the herbal preparation St John’s Wort (Hypericum perforatum) which is capable of decreasing tacrolimus blood levels.
Your doctor or pharmacist can tell you what to do if you are taking any of these medicines.
Effects on driving and operating machinery
- PHARMACOR TACROLIMUS may cause visual or nervous disturbances. If affected, do not drive or operate machinery.
Effects of food and alcohol
- Food reduces the absorption of PHARMACOR TACROLIMUS so the capsules should be taken at least 1 hour before a meal.
Using PHARMACOR TACROLIMUS
How much to take:
You can only get PHARMACOR TACROLIMUS from your doctor. Your dose will be calculated according to your weight, age, and medical condition. As your health and the function of your new liver or kidney, lung or heart can be affected by how much medicine you take, it is normal that your doctor collects samples of blood and urine at regular intervals. This is in order to test whether your medicine requires adjustment.
PHARMACOR TACROLIMUS should be taken in two doses (e.g. morning and evening). Take the capsule from the blister pack and swallow it whole with plenty of water. Do not use grapefruit juice as it contains substances that interfere with the action of PHARMACOR TACROLIMUS.
How to take it:
- PHARMACOR TACROLIMUS capsules should be taken at least 1 hour before a meal.
- You must never change the dose yourself even if you are feeling better. It is very important that you keep taking this medicine so that your body will not reject your new liver kidney, lung or heart.
- If you accidentally take a larger dose than recommended, tell your doctor immediately.
- If you do not understand the instructions provided with this medicine, ask your doctor or pharmacist for help.
If you forget to take it
- If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
- Do not take a double dose to make up for the dose you missed.
If you have missed more than one dose, or are not sure what to do, check with your doctor or pharmacist.
If you have trouble remembering when to take your medicine, ask your pharmacist for some hints.
If you have taken too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at your nearest hospital.
Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
Poisons Information Centre telephone numbers:
While you are taking PHARMACOR TACROLIMUS
Things you must do
- Always follow your doctor’s instructions carefully
- Tell your doctor if you become pregnant while taking PHARMACOR TACROLIMUS
- If you are about to start taking a new medicine, tell your doctor and pharmacist that you are taking PHARMACOR TACROLIMUS.
- PHARMACOR TACROLIMUS suppress your immune system by lowering your body's immune defence system. This increases your risk of skin cancer and other cancers while taking PHARMACOR TACROLIMUS. You should always protect yourself from the sun, wear sunscreen, a hat and protective clothing.
Things you must not do
- Do not take PHARMACOR TACROLIMUS to treat any other complaint unless your doctor says so.
- Do not give this medicine to anyone else, even if their symptoms seem similar to yours.
Side Effects
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some side effects.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you experience any of the following and they worry you:
- tiredness, lack of energy
- stomach upset, including nausea (feeling sick), vomiting, loss of appetite, diarrhoea, stomach cramps
- tremor (shaking)
- headache
- feeling depressed (sad)
- sleeping difficulties
- blurred vision or sensitive to light
- muscle cramps, tenderness or weakness
Tell your doctor immediately if you notice any of the following as you may need urgent medical care:
- signs of allergy such as rash, itching or hives on the skin; swelling of the face, lips, tongue or other part of the body; shortness of breath, wheezing or troubled breathing
- fever
- diabetes / increased blood sugar
- swelling, numbness or tingling (pins and needles) in your hands and feet
- constant "flu-like" symptoms such as chills, sore throat, aching joints, swollen glands, or any other signs of infection
- unusual bleeding or bruising
- high blood pressure
- palpitations, abnormal heart rhythms, chest pain
- new lumps or moles, or changes to existing moles, anywhere on the body
- swelling of the eyelids, hands or feet due to excess fluid
- a change in the amount of urine passed or in the number of times you urinate, pain on urinating, or other kidney problems.
- yellowing of the skin and/or eyes (jaundice) often accompanied by generally feeling unwell (for example, tiredness, lack of energy, loss of appetite, nausea and vomiting, pain in the abdomen)
- symptoms of anaemia, such as shortness of breath, tiredness or dizziness
- seizures (fits)
- buzzing or ringing in the ears, difficulty hearing
Other side effects not listed above may also occur in some people. Tell your doctor if you notice any other effects.
After using PHARMACOR TACROLIMUS
Storage
Use all the capsules within 12 months of opening the aluminium wrapper.
Keep PHARMACOR TACROLIMUS Capsules in the blisters until it is time to take them.
Keep PHARMACOR TACROLIMUS Capsules in a cool dry place where the temperature is below 25 degrees C.
Keep your medicines where children cannot reach them. A locked cupboard at least one-and-a-half metres (1.5 m) above the ground is a good place to store medicines.
Do not store PHARMACOR TACROLIMUS, or any other medicine, in the bathroom or near a sink. Do not leave medicines in the car or on window sills. Heat and dampness can destroy some medicines.
Disposal
If your doctor tells you to stop taking PHARMACOR TACROLIMUS Capsules, or your medicine has passed its expiry date, ask your pharmacist what to do with any medicine which may be left over.
Product Description
What it looks like
PHARMACOR TACROLIMUS
- PHARMACOR TACROLIMUS capsules 0.5 mg are yellow, packed in blister sheets of ten capsules and sealed in an aluminium wrapper (Pack size 100 capsules).
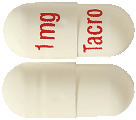
- PHARMACOR TACROLIMUS 1 mg capsules are white, packed in blister sheets of ten capsules and sealed in an aluminium wrapper (Pack size 100 capsules).
- PHARMACOR TACROLIMUS 5 mg capsules are greyish-red, packed in blister sheets of ten capsules and sealed in an aluminium wrapper (Pack size 50 capsules).
Ingredients
PHARMACOR TACROLIMUS Capsules contains:
- Hypromellose
- croscarmellose sodium
- lactose
- magnesium stearate
- gelatin
- water
- sodium lauryl sulfate
- titanium dioxide
- colorants: iron oxide yellow for 0.5 mg capsules and iron oxide red for 5 mg capsules
- printing ink: 0.5 mg and 1 mg capsules contains shellac and iron oxide red, 5 mg capsule contain shellac, potassium hydroxide and titanium dioxide.
Sponsor
Pharmacor Pty Ltd.
Suite 401, 7 oaks Avenue
Chatswood, NSW 2067
Australia
Australian Registration numbers:
PHARMACOR TACROLIMUS capsules 0.5 mg: 209272
PHARMACOR TACROLIMUS capsules 1 mg: 209266
PHARMACOR TACROLIMUS capsules 5 mg: 209267
Date of most recent amendment
October 2020
Published by MIMS December 2020



 Molecular formula: C44H69NO12.H2O.
Molecular formula: C44H69NO12.H2O.