What is in this leaflet
This leaflet answers some common questions about PIAX PLUS ASPIRIN 75/100 tablets.
It does not contain all of the available information. Some of the information it contains may not apply to you.
It does not take the place of talking to your doctor or pharmacist.
All medicines have benefits and risks. In deciding to give you PIAX PLUS ASPIRIN 75/100, your doctor has weighed the risks of you taking PIAX PLUS ASPIRIN 75/100 against the expected benefits it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Please read this leaflet carefully before you start taking PIAX PLUS ASPIRIN 75/100.
You may need to read it again.
What PIAX PLUS ASPIRIN 75/100 is used for
PIAX PLUS ASPIRIN 75/100 contains the medicines clopidogrel and aspirin. PIAX PLUS ASPIRIN 75/100 belongs to a group of medicines called anti-platelet medicines
Platelets are very small blood cells which clump together during blood clotting. By preventing this clumping, anti-platelet medicines reduce the chances of blood clots forming (a process called thrombosis).
PIAX PLUS ASPIRIN 75/100 is used to prevent blood clots forming in hardened blood vessels (a process known as atherothrombosis) which can lead to events such as stroke, heart attack or death.
You may have been prescribed PIAX PLUS ASPIRIN 75/100 to help prevent blood clots forming and to reduce the risk of heart attack, stroke or death, because you have suffered a severe type of chest pain called unstable angina, or had a heart attack.
Your doctor may have prescribed this medicine for another use. If you want more information, ask your doctor.
PIAX PLUS ASPIRIN 75/100 is only available on a doctor's prescription.
Before you take PIAX PLUS ASPIRIN 75/100
When you must not take it
Do not take PIAX PLUS ASPIRIN 75/100 if:
- you are allergic to clopidogrel, aspirin, salicylates, anti-inflammatories (Non-Steroidal Anti-Inflammatory Drugs) or any of the ingredients listed under 'Product Description' at the end of this leaflet.
- you have a medical condition that is causing bleeding such as a haemophilia, stomach ulcer or bleeding within your head or bowel.
- you suffer from severe liver or kidney disease.
- you have asthma, rhinitis or nasal polyps.
- you are breastfeeding or intend to breastfeed. PIAX PLUS ASPIRIN 75/100 passes into breast milk and, therefore, there is the possibility that the breastfed baby may be affected.
- the packaging shows signs of tampering.
- the expiry date on the pack has passed. If you use this product after the expiry date has passed, it may not work.
Do not take PIAX PLUS ASPIRIN 75/100 to treat any other complaint unless your doctor says it is safe. Do not give this medicine to anyone else.
PIAX PLUS ASPIRIN 75/100 is not recommended for children as its safety and effectiveness in children have not been established.
Before you start to take it
You must tell your doctor if:
- you have allergies to:
- any other medicines
- any other substances, such as foods, preservatives or dyes - you are pregnant or intend to become pregnant. Your doctor will discuss the possible risks and benefits of taking PIAX PLUS ASPIRIN 75/100 during pregnancy
- you are breastfeeding. You must not breastfeed while taking PIAX PLUS ASPIRIN 75/100
- you are planning to have an operation (including dental surgery) in the next two weeks. Your doctor will decide whether or not you need to stop PIAX PLUS ASPIRIN 75/100 prior to surgery
- if you have or have had any medical conditions, especially the following:
- bleeding disorders or blood clotting problems
- any illness or disability that was caused by bleeding, for example impaired sight or vision because of bleeding within the eye
- recent serious injury
- recent surgery (including dental surgery)
- any form of liver disease
- any recent history of stroke
- any form of kidney disease
- history of stomach ulcers or other problems with your digestive system
- inherited diseases causing galactose intolerance, glucose-galactose malabsorption
- lapp lactase deficiency
- glucose-6-phosphate dehydrogenase (G6PD) deficiency
- gout
- asthma or allergies
- allergic to other antiplatelet medicines (such as ticlopidine, prasugrel)
If you have not told your doctor about any of the above, tell him/her before you start taking PIAX PLUS ASPIRIN 75/100.
Some patients may not convert PIAX PLUS ASPIRIN 75/100 to its active form as well as other patients. These patients may not get the same benefit from PIAX PLUS ASPIRIN 75/100. Your doctor may advise you to go for tests to determine if PIAX PLUS ASPIRIN 75/100 will adequately work for you. Based on the test results, your doctor may consider alternative treatments for you.
Taking other medicines
Tell your doctor if you are taking any other medicines including any that you have bought without a prescription from a pharmacy, supermarket or healthfood shop.
Some medicines and PIAX PLUS ASPIRIN 75/100 may interfere with each other. These include:
- aspirin - PIAX PLUS ASPIRIN 75/100 already contains aspirin. Taking additional aspirin is not recommended. Please check with your doctor first
- clopidogrel - PIAX PLUS ASPIRIN 75/100 already contains clopidogrel. Taking additional clopidogrel is not recommended. Please check with your doctor first
- medicines that "thin the blood". The most common examples of these include heparins and warfarin. There are others so please check with your doctor
- non-steroidal anti-inflammatory drugs (NSAIDs) - medicines used to treat arthritis, period pain, aches and pains such as ibuprofen
- nicorandil - a medicine used to treat angina
- steroids e.g. hydrocortisone.
- bupropion
- diazepam
- some gout medicines
- some antidepressant medicines
- antiretrovirals e.g tenofovir
- ciprofloxacin, chloramphenicol, fluconazole and voriconazole - medicines used to treat infections
- varicella vaccine
- methotrexate - a medicine used to treat cancer or arthritis
- acetazolamide - a medicine used to treat glaucoma
- carbamazepine, oxcarbazepine, phenytoin and valproic acid - medicines used to treat epilepsy
- tolbutamide and chlorpropamide- a medicine used to treat diabetes
- tamoxifen - a medicine used to treat breast cancer
- levothyroxine - a medicine used to treat low thyroid activity
- fluvastatin - a medicine used to lower cholesterol
- ACE inhibitors or angiotensin receptor antagonists plus a thiazide diuretic. These medicines are used to treat high blood pressure. In some cases the medicines may be used together to treat other cardiovascular diseases
- medicines used to prevent gastric reflux - proton pump inhibitors (e.g. omeprazole)
- certain type of pain relief medicines called opiates
- rosuvastatin (used to lower your cholesterol level).
These medicines may be affected by PIAX PLUS ASPIRIN 75/100 or affect how well PIAX PLUS ASPIRIN 75/100 works.
Your doctor may need to change the amount of your medicines, or you may need to take different medicines.
If you are unsure about any medicine you are taking you should check with your doctor or pharmacist. They will have more information on medicines to be careful with or avoid while taking PIAX PLUS ASPIRIN 75/100.
Alcohol Consumption
The consumption of alcohol may affect how well PIAX PLUS ASPIRIN 75/100 works; it may increase blood loss and stomach irritation. Please ask your doctor for more information.
How to take PIAX PLUS ASPIRIN 75/100
How to take it
PIAX PLUS ASPIRIN 75/100 is to be used under medical supervision only.
Your doctor will tell you how many tablets to take each day. Take PIAX PLUS ASPIRIN 75/100 only as prescribed by your doctor and follow his or her directions carefully. They may differ from the information contained in this leaflet.
The usual dose of PIAX PLUS ASPIRIN 75/100 is one tablet daily.
PIAX PLUS ASPIRIN 75/100 contains 75 mg clopidogrel (as hydrogen sulfate) and 100 mg aspirin
You may receive a starting dose of 300 mg clopdiogrel, then one PIAX PLUS ASPIRIN 75/100 tablet daily. Take PIAX PLUS ASPIRIN 75/100 during or immediately after a meal. You should swallow the tablet with a glass of water.
Take PIAX PLUS ASPIRIN 75/100 at about the same time each day. Taking your tablet at the same time each day will have the best effect. It will also help you to remember when to take it.
You should take PIAX PLUS ASPIRIN 75/100 for as long as your doctor continues to prescribe it.
Should your doctor require you to take higher doses of aspirin you will be switched to separate tablets and no longer take PIAX PLUS ASPIRIN 75/100.
If you forget to take it
If you miss a dose, take it as soon as you remember. If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (Australia telephone 13 11 26), or go to the Emergency Department at your nearest hospital, if you think that you or anyone else may have taken too much PIAX PLUS ASPIRIN 75/100. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
While you are taking PIAX PLUS ASPIRIN 75/100
Things you must do
- take PIAX PLUS ASPIRIN 75/100 exactly as your doctor has prescribed, and have any blood tests promptly if your doctor orders them
- tell your doctor if you become pregnant while taking PIAX PLUS ASPIRIN 75/100.
- tell your doctor if you decide to breastfeed your baby. Your doctor may want to discuss this and change your medicine.
- tell your doctor that you are taking PIAX PLUS ASPIRIN 75/100 if you are about to start on any new medicine.
- tell all your doctors, dentists, nurses and pharmacists that you are taking PIAX PLUS ASPIRIN 75/100. PIAX PLUS ASPIRIN 75/100 may increase the risk of bleeding during an operation or some dental work. Therefore, treatment may need to be stopped before surgery. Your doctor will decide whether to stop PIAX PLUS ASPIRIN 75/100 and if so, how long before surgery or dental work.
- ask your doctor whether there are any activities you should avoid while taking PIAX PLUS ASPIRIN 75/100, for example certain sports.
Sometimes after an injury bleeding may occur inside your body without you knowing about it. - tell your doctor immediately if you are injured while taking PIAX PLUS ASPIRIN 75/100.
It may take longer than usual to stop bleeding while you are taking PIAX PLUS ASPIRIN 75/100. - tell your doctor immediately if you notice any of the following:
- abnormal bruising or bleeding
- abnormal nose bleeds
- bloody or black bowel motions
- red or purple blotches on your skin
- swelling of the face, lips, mouth, tongue or throat which may cause difficulty swallowing or breathing (see also 'Side effects' section)
Do not suddenly stop taking PIAX PLUS ASPIRIN 75/100 without telling your doctor.
Things to be careful of
Be careful driving or operating machinery until you know how PIAX PLUS ASPIRIN 75/100 affects you. As with other medicines, PIAX PLUS ASPIRIN 75/100 may cause faintness or dizziness in some people. Make sure you know how you react to PIAX PLUS ASPIRIN 75/100 before you drive a car or operate machinery, or do anything else that could be dangerous if you are faint or dizzy. If this occurs, do not drive. If you drink alcohol, faintness or dizziness may be worse.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking PIAX PLUS ASPIRIN 75/100 tablets. Like other medicines PIAX PLUS ASPIRIN 75/100 can cause some side effects. Most are likely to be minor and temporary. However, some may be serious and need medical attention.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you notice any of the following and they worry you:
- diarrhoea
- itching
- pain or stiffness in the joints
- ringing in the ears
- things taste different
- hunger
- trembling
- flushing
- a fast, pounding heart beat
Tell your doctor immediately if you notice any of the following:
- a severe reaction affecting the skin, blood and internal organs (DRESS)
- bloody or black bowel motions
- diarrhoea with blood, mucus, stomach pain and fever
- abdominal or stomach pain
- heartburn
- vomiting of blood or vomit that looks like coffee grounds
- coughing up blood
- blood in the urine
- blood in the eyes
- unusually heavy bleeding or oozing from cuts or wounds
- bleeding (including nose bleeds) or bruising more easily than normal
- unusually heavy or unexpected menstrual bleeding
- breast enlargement in men
- numbness (paralysis) or problems with co-ordination
- nausea or vomiting
- faintness or dizziness
- light-headedness or blurred vision
- slurred speech or other difficulty in speaking
- headache (severe and continuing)
- confusion or hallucinations
- fever or other signs of infection, such as a sore throat
- rash or hives
- chills, sweating or clammy skin
- fever, muscle weakness, loss of appetite and fatigue
- muscle pain
- weight loss
- anaemia (being tired and looking pale)
- red or purple spots visible through your skin
- itching, inflamed, cracking or red skin
- tightness of the chest, wheezing, coughing or difficulty breathing
- yellowing of the skin or the whites of the eyes, pale stools and dark urine with vomiting and stomach pain
- swelling of the face, lips, mouth, tongue or throat which may cause difficulty in swallowing or breathing
- oedema (build up of fluid in the body that can cause swelling).
These could be more serious side effects - you may need urgent medical attention.
Other side effects not listed above may also occur in some patients. Tell your doctor if you notice any other effects.
Do not be alarmed by this list of side effects. Most people do not experience any of them.
After taking PIAX PLUS ASPIRIN 75/100
Storage
Keep your tablets in the blister pack until it is time to take them. If you take your tablets out of the box or blister pack they will not keep well.
Keep PIAX PLUS ASPIRIN 75/100 in a cool, dry place where the temperature stays below 30°C. Heat and dampness can destroy some medicines. Do not leave PIAX PLUS ASPIRIN 75/100 in the car on hot days.
Do not store PIAX PLUS ASPIRIN 75/100 or any other medication in the bathroom or near a sink.
Keep PIAX PLUS ASPIRIN 75/100 where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking PIAX PLUS ASPIRIN 75/100, ask your pharmacist what to do with any tablets that are left over taking.
Product description
What it looks like
PIAX PLUS ASPIRIN 75/100 is available as:
PIAX PLUS ASPIRIN 75/100 75mg/100mg tablets:
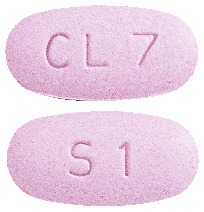
Mottled purple, oval, biconvex tablets debossed with 'CL7' on one side and 'S1' on the other.
AUST R 219059
Supplied in a box containing 30 tablets.
Active Ingredients
PIAX PLUS ASPIRIN 75/100 75mg/100mg: clopidogrel 75 mg, aspirin 100 mg
Other Ingredients
- lactose anhydrous
- microcrystalline cellulose
- crospovidone
- glycerol dibehenate
- hydrogenated castor oil
- Pigment blend PB-200001 PURPLE (109346)
PIAX PLUS ASPIRIN 75/100 contains sugars as lactose.
Sponsor
Medis Pharma Pty Ltd
Level 1, Epping Road
Macquarie Park, NSW 2113
Australia
Email: [email protected]
Distributor
PIAX PLUS® ASPIRIN 75/100 is distributed in Australia by:
Alphapharm Pty Ltd trading as Viatris
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.viatris.com.au
PIAX® PLUS is a Viatris company trade mark.
This leaflet was prepared in February 2024
Published by MIMS May 2024



 Clinically relevant adverse reactions not listed above pooled from CAPRIE, CURE, CLARITY and COMMIT studies with an incidence of ≥ 0.1% as well as all serious and clinically relevant adverse reactions are listed below according to the World Health Organization classification. Their frequency is defined using the following conventions: common: > 1/100 (1%) and < 1/10 (10%); uncommon: ≥ 1/1000 (0.1%) and < 1/100 (1%) and rare: ≥ 1/10,000 (0.01%) and < 1/1000 (0.1%).
Clinically relevant adverse reactions not listed above pooled from CAPRIE, CURE, CLARITY and COMMIT studies with an incidence of ≥ 0.1% as well as all serious and clinically relevant adverse reactions are listed below according to the World Health Organization classification. Their frequency is defined using the following conventions: common: > 1/100 (1%) and < 1/10 (10%); uncommon: ≥ 1/1000 (0.1%) and < 1/100 (1%) and rare: ≥ 1/10,000 (0.01%) and < 1/1000 (0.1%). The randomised, double blind, placebo controlled, 2 x 2 factorial design COMMIT trial included 45,852 patients presenting within 24 hours of the onset of the symptoms of suspected myocardial infarction with supporting ECG abnormalities (i.e. ST elevation, ST depression or left bundle branch block). Patients were randomised to receive clopidogrel (75 mg/day) or placebo, in combination with aspirin (162 mg/day), for 28 days or until hospital discharge, whichever came first.
The randomised, double blind, placebo controlled, 2 x 2 factorial design COMMIT trial included 45,852 patients presenting within 24 hours of the onset of the symptoms of suspected myocardial infarction with supporting ECG abnormalities (i.e. ST elevation, ST depression or left bundle branch block). Patients were randomised to receive clopidogrel (75 mg/day) or placebo, in combination with aspirin (162 mg/day), for 28 days or until hospital discharge, whichever came first.

 The benefit associated with clopidogrel on the combined endpoint was consistent across age, gender and with or without fibrinolytics and was observed as early as 24 hours.
The benefit associated with clopidogrel on the combined endpoint was consistent across age, gender and with or without fibrinolytics and was observed as early as 24 hours.

 Consistent with the above results, in a meta-analysis including 6 studies of 335 clopidogrel treated subjects at steady state, it was shown that active metabolite exposure was decreased by 28% for intermediate metabolisers, and 72% for poor metabolisers while platelet aggregation inhibition (5 microM ADP) was decreased with differences in IPA of 5.9% and 21.4%, respectively, when compared to extensive metabolisers.
Consistent with the above results, in a meta-analysis including 6 studies of 335 clopidogrel treated subjects at steady state, it was shown that active metabolite exposure was decreased by 28% for intermediate metabolisers, and 72% for poor metabolisers while platelet aggregation inhibition (5 microM ADP) was decreased with differences in IPA of 5.9% and 21.4%, respectively, when compared to extensive metabolisers. Molecular formula: C16H16ClNO2S.H2SO4.
Molecular formula: C16H16ClNO2S.H2SO4. Molecular formula: C9H8O4.
Molecular formula: C9H8O4.