WHAT IS IN THIS LEAFLET
This leaflet answers some common questions about Pioglitazone Sandoz.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risk of you taking this medicine against the benefits it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet. You may need to read it again.
WHAT PIOGLITAZONE SANDOZ IS USED FOR
Pioglitazone Sandoz helps to control the level of glucose in your blood when you have type 2 diabetes. This is the 'adult onset' type of diabetes and is controlled by diet, certain oral medications and occasionally insulin.
This medicine is used:
- to improve the action of the body's naturally produced insulin.
- in the management of type 2 diabetes not controlled by diet.
- alone (when diet and exercise is not enough to treat your diabetes) or together with other anti-diabetic medicines.
It contains the active ingredients Pioglitazone hydrochloride.
Pioglitazone hydrochloride belongs to a group of medicines called glitazones.
It works by decreasing insulin resistance.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
This medicine is not addictive.
This medicine is available only with a doctor's prescription.
The use of Pioglitazone Sandoz has not been studied in children.
BEFORE YOU TAKE PIOGLITAZONE SANDOZ
When you must not take it
Do not take this medicine if you have an allergy to:
- Pioglitazone hydrochloride, the active ingredient, or to any of the other ingredients listed at the end of this leaflet under Product Description.
- any other similar medicines (Rosiglitazone maleate).
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin.
Do not take this medicine if you have or have had any of the following medical conditions:
- heart failure requiring treatment.
Talk to your doctor if you have heart failure - type 1 diabetes or diabetic ketoacidosis
- bladder cancer
- hepatic impairment.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you have or have had any of the following medical conditions:
- red coloured urine
- heart disease with shortness of breath after minimal physical activity
- heart disease with severe symptoms at rest
- swelling of hands, ankles or feet
- bladder cancer or symptoms associated with bladder cancer such as blood in the urine (hematuria) often accompanied by pain and burning or, sudden urges to urinate
- problems with your liver
- problems with your kidneys that requires dialysis. Pioglitazone Sandoz is not recommended for use if you are on dialysis
- some women who do not have monthly periods and have not been through menopause may restart their periods when taking Pioglitazone Sandoz. These women may be at increased risk of pregnancy
- bone fractures, usually in the hand, upper arm or foot, have been seen in some patients when taking Pioglitazone Sandoz. Talk to your doctor for advice on how to keep your bones healthy.
Tell your doctor if you are pregnant or plan to become pregnant or are breast-feeding. Like most medicines, Pioglitazone Sandoz is not recommended for use during pregnancy. If there is a need to consider Pioglitazone Sandoz during your pregnancy, your doctor will discuss with you the benefits and risks of taking Pioglitazone Sandoz. It is recommended that you do not breast-feed while taking Pioglitazone Sandoz, as it is not known whether Pioglitazone Sandoz passes into breast milk.
Tell your doctor if you are using another medicine for diabetes. Pioglitazone Sandoz can enhance the action of other medicines. You may be at risk of low blood sugar (hypoglycaemia). If this happens, your doctor may need to adjust the dose of your other medicines.
Tell your doctor if you suffer from lactose intolerance (because Pioglitazone Sandoz tablets contain lactose).
If you have not told your doctor about any of the above, tell him/her before you start taking Pioglitazone Sandoz tablets.
Taking other medicines
Tell your doctor if you are taking any other medicine, including any that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and Pioglitazone Sandoz may interfere with each other. These include:
- gemfibrozil, a medicine used to lower high cholesterol
- other antidiabetic drugs such as glibenclamide, gliclazide, insulin, metformin, tolbutamide and chlorpropamide
- oral contraceptives
- rifampicin, an antibiotic drug.
These medicines may be affected by Pioglitazone Sandoz or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
HOW TO TAKE PIOGLITAZONE SANDOZ
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions, ask your doctor or pharmacist for help.
How much to take
Your doctor will tell you how many Pioglitazone Sandoz tablets you should take.
The standard dose for this medicine is usually in the range of 15 mg to 45 mg per day.
Your doctor may have prescribed a different dose.
Ask your doctor or pharmacist if you are unsure of the correct dose for you. They will tell you exactly how much to take.
Follow the instructions they give you.
If you take the wrong dose, Pioglitazone Sandoz may not work as well and your problem may not improve.
The usual starting dose is one tablet each day as advised by your doctor. Depending on your response, your doctor may increase or decrease your dose in order to find the appropriate dose for your condition.
How to take it
Swallow the tablet whole with a full glass of water.
When to take Pioglitazone Sandoz
Take your medicine at about the same time each day. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
It does not matter whether you take Pioglitazone Sandoz with food or on an empty stomach.
How long to take Pioglitazone Sandoz
Continue taking your medicine for as long as your doctor tells you.
This medicine helps to control your condition, but does not cure it. It is important to keep taking your medicine even if you feel well.
If you forget to take it
Take your dose as soon as you remember, and continue to take it as you would normally.
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Do not take a double dose to make up for the dose that you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone Australia 13 11 26 or New Zealand 0800 POISON or 0800 764766) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much Pioglitazone Sandoz. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
WHILE YOU ARE USING PIOGLITAZONE SANDOZ
Things you must do
It is important that you remember to take Pioglitazone Sandoz daily and at the dose prescribed by your doctor.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking Pioglitazone Sandoz.
Tell any other doctors, dentists and pharmacists who treat you that you are taking this medicine.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking this medicine. It may affect other medicines used during surgery.
If you become pregnant while taking this medicine, tell your doctor immediately.
If you are about to have any blood tests, tell your doctor that you are taking this medicine. It may interfere with the results of some tests.
Keep all of your doctor's appointments so that your progress can be checked. Your doctor may do some tests from time to time to make sure the medicine is working and to prevent unwanted side effects.
Tell your doctor if you have gained weight since taking Pioglitazone Sandoz. Weight gain can be associated with improved blood sugar control however; it may also be a symptom of heart failure.
Things you must not do
Do not take Pioglitazone Sandoz to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or lower the dosage without checking with your doctor. If you stop taking it suddenly, your condition may worsen or you may have unwanted side effects.
Things to be careful of
Pioglitazone Sandoz alone is unlikely to affect your ability to drive or operate machinery. However, be careful to avoid hypoglycaemia whilst driving or operating machinery if using Pioglitazone Sandoz in combination with other diabetes medicines.
SIDE EFFECTS
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Pioglitazone Sandoz.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
This medicine helps most people with type 2 diabetes not controlled by diet, but it may have unwanted side effects in some people.
Some side effects may be related to the dose of Pioglitazone Sandoz. Accordingly, it is important that you tell your doctor as soon as possible about any unwanted effects. Your doctor may then decide to adjust the dose of Pioglitazone Sandoz you are taking.
Do not be alarmed by the following list of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
A few patients have experienced the following side effects whilst taking Pioglitazone Sandoz:
- a small increase in weight
- low blood sugar levels (hypoglycaemia). This occurs more often when Pioglitazone Sandoz is taken with a sulfonylurea or insulin
- heart failure which may show as localised swelling of the ankles, feet and hands (oedema) and/or fluid in the lungs (pulmonary oedema). This has been reported in clinical trials mainly in patients who are taking pioglitazone in combination with insulin
- increased risk of fracture
- macular oedema (an eye disorder that can affect vision)
- altered or impaired liver function.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- weight gain
- signs of hypoglycaemia which may include weakness, trembling or shaking, sweating, light-headedness, headache, dizziness, lack of concentration, tearfulness or crying, irritability, hunger, numbness around the lips and fingers
- eye problems including blurred or double vision.
These are more common side effects of the medicine.
Tell your doctor as soon as possible if you notice any of the following:
- dark urine or pale stools, yellowing of the skin or eyes, severe cramps of the stomach, nausea or vomiting, loss of weight, tiredness
- shortness of breath when at rest or after minimal physical activity with swelling of legs, feet and hands, rapid increase in weight
- blood in the urine often accompanied by pain and burning, these can be symptoms of bladder cancer.
The above list includes serious side effects that may require medical attention. Serious side effects are rare.
Tell your doctor or pharmacist if you notice anything else that is making you feel unwell. Other side effects not listed above may also occur in some people.
AFTER TAKING PIOGLITAZONE SANDOZ
Storage
Keep your medicine in the original container.
If you take it out of its original container it may not keep well.
Keep your medicine in a cool dry place where the temperature stays below 30°C.
Do not store Pioglitazone Sandoz or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car.
Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
PRODUCT DESCRIPTION
What it looks like
Pioglitazone Sandoz comes in three types of tablets:
Pioglitazone Sandoz 15 mg - white, round tablet, with imprint "PGT 15" on one side.
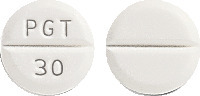
Pioglitazone Sandoz 30 mg - white, round tablet, with imprint "PGT 30" on one side and with score line on both sides.
Pioglitazone Sandoz 45 mg - white, round tablet, with imprint "PGT 45" on one side.
Available in blister packs and bottles of 28 tablets.
Not all strengths or presentations may be marketed.
Ingredients
Active ingredients:
- Pioglitazone Sandoz 15 mg - 15 mg Pioglitazone (as hydrochloride)
- Pioglitazone Sandoz 30 mg - 30 mg Pioglitazone (as hydrochloride)
- Pioglitazone Sandoz 45 mg - 45 mg Pioglitazone (as hydrochloride)
Inactive ingredients:
- Lactose monohydrate
- hyprolose
- carmellose calcium
- magnesium stearate
This medicine does not contain gluten, tartrazine or any other azo dyes.
Supplier
Sandoz Pty Ltd
ABN 60 075 449 553
54 Waterloo Road
Macquarie Park, NSW 2113
Australia
Tel: 1800 726 369
Novartis New Zealand Ltd
PO Box 99102
Newmarket
Auckland 1149
New Zealand
Tel: 0800 354 335
This leaflet was prepared in January 2019.
Australian Register Number:
15 mg tablets: AUST R 162223 (blisters)
30 mg tablets: AUST R 162222 (blisters)
45 mg tablets: AUST R 162225 (blisters)
Published by MIMS March 2019



 In some cases weight increase may be a symptom of cardiac failure, therefore weight should be closely monitored. Part of the treatment of diabetes is dietary control. Patients should be advised to adhere strictly to a calorie-controlled diet.
In some cases weight increase may be a symptom of cardiac failure, therefore weight should be closely monitored. Part of the treatment of diabetes is dietary control. Patients should be advised to adhere strictly to a calorie-controlled diet.

 In the PROactive study, which involved a high risk population of patients with pre-existing macrovascular disease, treatment emergent adverse events that occurred more often in the pioglitazone group compared to placebo group were oedema (26.4 and 15.1%, respectively), hypoglycaemia (27.2 and 18.8%, respectively) and cardiac failure, including serious and non-serious cases (12.6% and 8.7% respectively).
In the PROactive study, which involved a high risk population of patients with pre-existing macrovascular disease, treatment emergent adverse events that occurred more often in the pioglitazone group compared to placebo group were oedema (26.4 and 15.1%, respectively), hypoglycaemia (27.2 and 18.8%, respectively) and cardiac failure, including serious and non-serious cases (12.6% and 8.7% respectively). The study population included patients not previously treated with antidiabetic medication (naive 31%) and patients who were receiving antidiabetic medication at the time of study enrolment (previously treated 69%). The data for the naive and previously treated patient subsets are shown in Table 5. This run-in period was associated with little change in HbA1c and FBG values from screening to baseline for the naive patients. However, for the previously treated group, washout from previous antidiabetic medication resulted in deterioration of glycaemic control and increases in HbA1c and FBG. With pioglitazone, while most patients in the previously treated group had a decrease from baseline in HbA1c and FBG, in many cases the values did not return to screening levels by the end of the study. The study design did not permit the evaluation of patients who switched directly to pioglitazone from another antidiabetic agent. Please see Table 5.
The study population included patients not previously treated with antidiabetic medication (naive 31%) and patients who were receiving antidiabetic medication at the time of study enrolment (previously treated 69%). The data for the naive and previously treated patient subsets are shown in Table 5. This run-in period was associated with little change in HbA1c and FBG values from screening to baseline for the naive patients. However, for the previously treated group, washout from previous antidiabetic medication resulted in deterioration of glycaemic control and increases in HbA1c and FBG. With pioglitazone, while most patients in the previously treated group had a decrease from baseline in HbA1c and FBG, in many cases the values did not return to screening levels by the end of the study. The study design did not permit the evaluation of patients who switched directly to pioglitazone from another antidiabetic agent. Please see Table 5. Pioglitazone has been shown to reduce total plasma triglycerides and free fatty acids and to increase HDL cholesterol levels. LDL cholesterol levels remain unchanged. In a 26 week, placebo-controlled, dose-ranging study, mean triglyceride levels decreased in the pioglitazone 15, 30 and 45 mg dose groups compared to a mean increase in the placebo group. Mean HDL levels increased to a greater extent in the pioglitazone treated patients than in the placebo-treated patients. There were no consistent differences for LDL and total cholesterol in pioglitazone treated patients compared with placebo (see Table 6).
Pioglitazone has been shown to reduce total plasma triglycerides and free fatty acids and to increase HDL cholesterol levels. LDL cholesterol levels remain unchanged. In a 26 week, placebo-controlled, dose-ranging study, mean triglyceride levels decreased in the pioglitazone 15, 30 and 45 mg dose groups compared to a mean increase in the placebo group. Mean HDL levels increased to a greater extent in the pioglitazone treated patients than in the placebo-treated patients. There were no consistent differences for LDL and total cholesterol in pioglitazone treated patients compared with placebo (see Table 6). In a separate 24 week study, 260 patients with type 2 diabetes were randomised to one of two forced-titration pioglitazone treatment arms (final doses 30 or 45 mg), or a mock titration placebo arm. In one pioglitazone treatment group, patients received an initial dose of 7.5 mg once daily. After four weeks, the dose was increased to 15 mg once daily and after another four weeks, the dose was increased to 30 mg once daily for the remainder of the study (16 weeks). In the second pioglitazone treatment group, patients received an initial dose of 15 mg once daily and were titrated to 30 mg once daily and 45 mg once daily in a similar manner. Treatment with pioglitazone, as described, produced statistically significant improvements in HbA1c and FBG at endpoint compared with placebo (see Table 7).
In a separate 24 week study, 260 patients with type 2 diabetes were randomised to one of two forced-titration pioglitazone treatment arms (final doses 30 or 45 mg), or a mock titration placebo arm. In one pioglitazone treatment group, patients received an initial dose of 7.5 mg once daily. After four weeks, the dose was increased to 15 mg once daily and after another four weeks, the dose was increased to 30 mg once daily for the remainder of the study (16 weeks). In the second pioglitazone treatment group, patients received an initial dose of 15 mg once daily and were titrated to 30 mg once daily and 45 mg once daily in a similar manner. Treatment with pioglitazone, as described, produced statistically significant improvements in HbA1c and FBG at endpoint compared with placebo (see Table 7). For patients who had not been previously treated with antidiabetic medication (24%), mean values at screening were 10.1% for HbA1c and 13.22 mmol/L for FBG. At baseline, mean HbA1c was 10.2% and mean FBG was 13.5 mmol/L. Compared with placebo, treatment with pioglitazone titrated to a final dose of 30 and 45 mg resulted in reductions from baseline in mean HbA1c of 2.3 and 2.6% and mean FBG of 3.5 and 5.28 mmol/L, respectively. For patients who had been previously treated with antidiabetic medication (76%), this medication was discontinued at screening. Mean values at screening were 9.4% for HbA1c and 12 mmol/L for FBG. At baseline, mean HbA1c was 10.7% and mean FBG was 16.11 mmol/L. Compared with placebo, treatment with pioglitazone titrated to a final dose of 30 and 45 mg resulted in reductions from baseline in mean HbA1c of 1.3 and 1.4% and mean FBG of 3.06 and 3.33 mmol/L, respectively. For many previously treated patients, HbA1c and FBG had not returned to screening levels by the end of the study.
For patients who had not been previously treated with antidiabetic medication (24%), mean values at screening were 10.1% for HbA1c and 13.22 mmol/L for FBG. At baseline, mean HbA1c was 10.2% and mean FBG was 13.5 mmol/L. Compared with placebo, treatment with pioglitazone titrated to a final dose of 30 and 45 mg resulted in reductions from baseline in mean HbA1c of 2.3 and 2.6% and mean FBG of 3.5 and 5.28 mmol/L, respectively. For patients who had been previously treated with antidiabetic medication (76%), this medication was discontinued at screening. Mean values at screening were 9.4% for HbA1c and 12 mmol/L for FBG. At baseline, mean HbA1c was 10.7% and mean FBG was 16.11 mmol/L. Compared with placebo, treatment with pioglitazone titrated to a final dose of 30 and 45 mg resulted in reductions from baseline in mean HbA1c of 1.3 and 1.4% and mean FBG of 3.06 and 3.33 mmol/L, respectively. For many previously treated patients, HbA1c and FBG had not returned to screening levels by the end of the study. For patients who had not been previously treated with antidiabetic medication (40%), mean values at screening were 10.3% for HbA1c and 13.33 mmol/L for FBG. At baseline, mean HbA1c was 10.4% and mean FBG was 14.11 mmol/L. Compared with placebo, treatment with pioglitazone 30 mg resulted in reductions from baseline in mean HbA1c of 1.0% and mean FBG of 3.44 mmol/L. For patients who had been previously treated with antidiabetic medication (60%), this medication was discontinued at screening. Mean values at screening were 9.4% for HbA1c and 12 mmol/L for FBG. At baseline, mean HbA1c was 10.6% and mean FBG was 15.94 mmol/L. Compared with placebo, treatment with pioglitazone 30 mg resulted in reductions from baseline in mean HbA1c of 1.3% and mean FBG of 2.56 mmol/L. For many previously treated patients, HbA1c and FBG had not returned to screening levels by the end of the study.
For patients who had not been previously treated with antidiabetic medication (40%), mean values at screening were 10.3% for HbA1c and 13.33 mmol/L for FBG. At baseline, mean HbA1c was 10.4% and mean FBG was 14.11 mmol/L. Compared with placebo, treatment with pioglitazone 30 mg resulted in reductions from baseline in mean HbA1c of 1.0% and mean FBG of 3.44 mmol/L. For patients who had been previously treated with antidiabetic medication (60%), this medication was discontinued at screening. Mean values at screening were 9.4% for HbA1c and 12 mmol/L for FBG. At baseline, mean HbA1c was 10.6% and mean FBG was 15.94 mmol/L. Compared with placebo, treatment with pioglitazone 30 mg resulted in reductions from baseline in mean HbA1c of 1.3% and mean FBG of 2.56 mmol/L. For many previously treated patients, HbA1c and FBG had not returned to screening levels by the end of the study.
 The criteria for assessing bioequivalence for pioglitazone were standard, i.e. 90% confidence intervals around the exponential of the difference between least-squares means of test and reference products for ln-transformed AUCt, AUCi and Cmax were to be between 80% and 125%.
The criteria for assessing bioequivalence for pioglitazone were standard, i.e. 90% confidence intervals around the exponential of the difference between least-squares means of test and reference products for ln-transformed AUCt, AUCi and Cmax were to be between 80% and 125%.
