1 Name of Medicine
Alpelisib.
2 Qualitative and Quantitative Composition
Active substance.
All Piqray tablets contain alpelisib.
Piqray 50 mg tablet.
Each tablet contains 50 mg of alpelisib.
Piqray 150 mg tablet.
Each tablet contains 150 mg of alpelisib.
Piqray 200 mg tablet.
Each tablet contains 200 mg of alpelisib.
Excipients.
For the list of excipients, see Section 6.1 List of Excipients.3 Pharmaceutical Form
Film coated tablets.
Piqray 50 mg tablet.
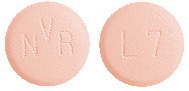
Light pink, unscored, round and curved with bevelled edges, imprinted with "L7" on one side and "NVR" on the other side.
Piqray 150 mg tablet.
Pale red, unscored, ovaloid and curved with bevelled edges, imprinted with "UL7" on one side and "NVR" on the other side.
Piqray 200 mg tablet.
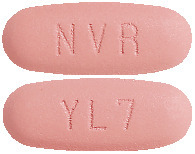
Light red, unscored, ovaloid and curved with bevelled edges, imprinted with "YL7" on one side and "NVR" on the other side.4.1 Therapeutic Indications
Piqray in combination with fulvestrant, is indicated for the treatment of postmenopausal women, and men, with hormone receptor positive, HER2-negative, advanced or metastatic breast cancer with a PIK3CA mutation as detected by a validated test following progression on or after an endocrine-based regimen.
4.2 Dose and Method of Administration
Treatment with Piqray should be initiated by a physician experienced in the use of anticancer therapies.
Patients with HR positive, HER2 negative advanced breast cancer should be selected for treatment with Piqray, based on the presence of a PIK3CA mutation in tumour or plasma specimens, using a validated test. If a mutation is not detected in a plasma specimen, test tumour tissue if available.
The safety and efficacy of alpelisib in combination with a GnRH agonist in pre- or peri-menopausal women has not been established.
There was no treatment benefit demonstrated in patients without PIK3CA mutations, in the phase III clinical study (see Section 5.1 Pharmacodynamic Properties).
Adult dose.
Recommended dosage.
The recommended dose of Piqray is 300 mg (two 150 mg film-coated tablets) taken orally, once daily. Piqray should be taken immediately following food, at approximately the same time each day (see Section 5.1 Pharmacodynamic Properties; Section 4.5 Interactions with Other Medicines and Other Forms of Interactions). The maximum recommended daily dose of Piqray is 300 mg. If patient vomits after taking the Piqray dose, the patient should not take an additional dose on that day, and should resume the usual dosing schedule the next day, at the usual time.
When co-administered with Piqray, the recommended dose of fulvestrant is 500 mg administered intramuscularly on days 1, 15 and, 29, and once monthly thereafter. Please refer to the full product information of fulvestrant.
Treatment should continue as long as clinical benefit is observed or until unacceptable toxicity occurs. Dosing modifications may be necessary to improve tolerability.
Missed dose.
If a dose of Piqray is missed, it can be taken immediately following food and within 9 hours after the time it is usually administered. After more than 9 hours, the missed dose should be skipped for that day. On the next day, Piqray should be taken at its usual time.
Dose modifications.
The recommended daily dose of Piqray is 300 mg. Management of severe or intolerable adverse drug reactions may require temporary dosing interruption, reduction, and/or discontinuation of Piqray. If dosing reduction is required, the dosing reduction guidelines for adverse drug reactions (ADRs) are listed in Table 1. A maximum of 2 dosing reductions are recommended, after which the patient should be discontinued from treatment with Piqray. Dosing reduction should be based on worst preceding toxicity.
 Tables 2, 3, 4 and 5 summarise recommendations for dosing interruption, reduction or discontinuation of Piqray in the management of specific ADRs. Clinical judgment of the treating physician, including confirmation of laboratory values if deemed necessary, should guide the management plan of each patient based on the individual benefit/risk assessment for treatment with Piqray.
Tables 2, 3, 4 and 5 summarise recommendations for dosing interruption, reduction or discontinuation of Piqray in the management of specific ADRs. Clinical judgment of the treating physician, including confirmation of laboratory values if deemed necessary, should guide the management plan of each patient based on the individual benefit/risk assessment for treatment with Piqray.
Hyperglycaemia.
Consultation with a Healthcare Professional (HCP) with experience in the management of hyperglycemia should be considered and lifestyle changes as per local guidelines, including exercise and dietary advice should be recommended/reinforced (e.g. small frequent meals, low carbohydrate, high fiber, low processed food intake, three macronutrient balanced meals and 2 optional small snacks rather than one large meal).
Premedication with metformin may decrease the incidence and severity of hyperglycemia when metformin is initiated 7 days prior to the start of treatment with Piqray and metformin dose titrated to the maximum tolerated dose. Patient risk factors for hyperglycaemia, gastrointestinal tolerability and the clinical situation should be considered before initiating metformin premedication. See Section 4.4 Special Warnings and Precautions for Use; Section 4.8 Adverse Effects (Undesirable Effects).
In patients with risk factors for hyperglycaemia, monitor fasting glucose more closely and as clinically indicated (see Section 4.4 Special Warnings and Precautions for Use). See Table 2.

Rash.
Oral antihistamine administration may be considered prophylactically, at the time of initiation of treatment with Piqray. Based on the severity of rash, Piqray may require dose interruption, reduction, or discontinuation as described in Table 3 (see Section 4.8 Adverse Effects (Undesirable Effects)).

Diarrhoea.
See Table 4 for dosing modifications and management guidelines.

Other toxicities.
See Table 5 for the dosing modification and management for other toxicities (excluding hyperglycaemia and rash).

Fulvestrant.
For dose modification guidelines in the event of toxicity or for any other relevant safety information, refer to the fulvestrant product information.
Special populations.
Renal impairment.
Based on population pharmacokinetic analysis, no dose adjustment is necessary in patients with mild or moderate renal impairment (see Section 5.2 Pharmacokinetic Properties). Caution should be used in patients with severe renal impairment as there is no experience with Piqray in this population (see Section 5.2 Pharmacokinetic Properties).
Hepatic impairment.
Based on a hepatic impairment study in non-cancer subjects with impaired hepatic function, no dose adjustment is necessary in patients with mild, moderate and severe hepatic impairment (Child-Pugh class A, B or C, respectively) (see Section 5.2 Pharmacokinetic Properties).
Refer to the product information of fulvestrant for dose modifications related to hepatic impairment.
Paediatric use.
The safety and efficacy of Piqray in paediatric patients have not been established.
Use in the elderly.
No dosage regimen adjustment is required in patients 65 years or above (see Section 5.2 Pharmacokinetic Properties).
Administration.
Piqray tablets should be swallowed whole (tablets should not be chewed, crushed or split prior to swallowing). Tablets that are broken, cracked, or otherwise not intact should not be ingested.4.3 Contraindications
Piqray is contraindicated in patients with hypersensitivity to the active substance or any of the excipients (see Section 6.1 List of Excipients).
4.4 Special Warnings and Precautions for Use
Hypersensitivity (including anaphylactic reaction).
Serious hypersensitivity reactions (including anaphylactic reaction and anaphylactic shock), manifested by symptoms including, but not limited to, dyspnoea, flushing, rash, fever or tachycardia were reported in patients treated with Piqray in clinical studies (see Section 4.8 Adverse Effects (Undesirable Effects)). Angioedema has been reported in the post marketing setting in patients treated with Piqray (see Section 4.8 Adverse Effects (Undesirable Effects)). Piqray should be permanently discontinued and should not be re-introduced in patients with serious hypersensitivity reactions. Appropriate treatment should be promptly initiated.
Severe cutaneous reactions.
Severe cutaneous reactions have been reported with Piqray. In the Phase III clinical study, Stevens-Johnson syndrome (SJS) and erythema multiforme (EM) were reported in 1 (0.4%) and 3 (1.1%) patients, respectively. Drug reaction with eosinophilia and systemic symptoms (DRESS) has been reported in the post marketing setting (see Section 4.8 Adverse Effects (Undesirable Effects)). Piqray treatment should not be initiated in patients with history of severe cutaneous reactions.
Patients should be advised of the signs and symptoms of severe cutaneous reactions (e.g. a prodrome of fever, flu-like symptoms, mucosal lesions or progressive skin rash). If signs or symptoms of severe cutaneous reactions are present, Piqray should be interrupted until the etiology of the reaction has been determined. A consultation with dermatologist is recommended. If a severe cutaneous reaction is confirmed, Piqray should be permanently discontinued. Piqray should not be reintroduced in patients who have experienced previous severe cutaneous reactions. If a severe cutaneous reaction is not confirmed, Piqray may require treatment interruption, dose reduction, or treatment discontinuation as described in Table 3 Dose modification and management for rash (see Section 4.2 Dose and Method of Administration).
Hyperglycaemia.
Severe hyperglycaemia, in some cases associated with hyperglycaemic hyperosmolar nonketotic syndrome (HHNKS) or ketoacidosis, has been observed in patients treated with Piqray. Some cases of ketoacidosis with fatal outcome have been reported in the post marketing setting.
Hyperglycaemia was reported in 64.8% of patients treated with Piqray in the phase III clinical study. Grade 2 (FPG 8.9 - 13.9 mmol/L), 3 (FPG > 13.9 - 27.8 mmol/L) or 4 (FPG > 27.8 mmol/L) hyperglycaemia were reported in 15.8%, 33.1% and 3.9% of patients, respectively, in phase III clinical study. In the phase III clinical study, patients with a history of diabetes mellitus intensified anti-diabetic medication(s) while on treatment with Piqray; therefore, these patients require monitoring and possibly intensified anti-diabetic treatment. Patients with poor glycaemic control may be at a higher risk of developing severe hyperglycaemia and associated complications. Patients with risk factors for hyperglycaemia such as obesity (BMI ≥ 30), elevated FPG or HbA1c at or above the upper limit of normal, or age ≥ 75 are at a higher risk of developing severe hyperglycaemia. The schedule for monitoring fasting glucose is presented in Table 6.
Patients should be advised of the signs and symptoms of hyperglycaemia (e.g. excessive thirst, urinating more often than usual or higher amount of urine than usual, and increased appetite with weight loss).
The safety of Piqray in patients with Type 1 and uncontrolled Type 2 diabetes has not been established as these patients were excluded from the Phase III clinical study.
 In the phase III clinical study, in patients with hyperglycaemia, 163/187 (87.2%) were managed with anti-diabetic medication and 142/187 (75.9%) reported use of metformin as single agent or in combination with other anti-diabetic medication. The maximum dose of metformin recommended in phase III clinical study was 2000 mg per day.
In the phase III clinical study, in patients with hyperglycaemia, 163/187 (87.2%) were managed with anti-diabetic medication and 142/187 (75.9%) reported use of metformin as single agent or in combination with other anti-diabetic medication. The maximum dose of metformin recommended in phase III clinical study was 2000 mg per day.
In patients with hyperglycaemia of at least Grade 2 (FPG 8.9 - 13.9 mmol/L), median time to improvement by at least 1 Grade of the first event was 8 days (95% CI of 8 to 10 days). In all patients with elevated FPG, who continued fulvestrant treatment after discontinuing Piqray, all FPG levels returned to baseline (normal).
Premedication with metformin may decrease the incidence and severity of hyperglycaemia when metformin is initiated 7 days prior to the start of treatment with Piqray, but increases the incidence of nausea and vomiting and the incidence and severity of diarrhoea. See Section 4.2 Dose and Method of Administration; Section 4.8 Adverse Effects (Undesirable Effects).
Based on the severity of the hyperglycaemia, Piqray may require dose interruption, reduction, or discontinuation as described in Table 2 Dose modification and management for hyperglycaemia (see Section 4.2 Dose and Method of Administration).
Pneumonitis.
Pneumonitis including serious cases of pneumonitis/acute interstitial lung disease have been reported in Piqray treated patients in clinical studies. Patients should be advised to promptly report any new or worsening respiratory symptoms. In patients who have new or worsening respiratory symptoms or are suspected to have developed pneumonitis, Piqray treatment should be interrupted immediately and the patient should be evaluated for pneumonitis. A diagnosis of non-infectious pneumonitis should be considered in patients presenting with non-specific respiratory signs and symptoms such as hypoxia, cough, dyspnoea, or interstitial infiltrates on radiologic exams and in whom infectious, neoplastic, and other causes have been excluded by means of appropriate investigations. Piqray should be permanently discontinued in all patients with confirmed pneumonitis.
Diarrhoea or colitis.
Severe diarrhoea and clinical consequences, such as dehydration, and acute kidney injury and hypovolaemic shock, have been reported during treatment with Piqray in clinical studies (see Section 4.8 Adverse Effects (Undesirable Effects)). In the phase III clinical study, Grade 2 and 3 diarrhoea was reported in 18.3% and 6.7% of patients, respectively. There were no reported cases of Grade 4 diarrhoea. Among patients with Grade 2 or 3 diarrhoea, median time to onset was 46 days (range: 1 to 442 days).
Colitis has been reported in the post marketing setting in patients treated with Piqray (see Section 4.8 Adverse Effects (Undesirable Effects)).
In the phase III clinical study, dose reductions of Piqray were required in 6% of patients and 2.8% of patients permanently discontinued Piqray due to diarrhoea.
Patients should be monitored for diarrhoea and additional symptoms of colitis, such as abdominal pain and mucus or blood in stool. Based on the severity of the diarrhoea or colitis, Piqray may require dose interruption, reduction, or discontinuation as described in Table 4 (see Section 4.2 Dose and Method of Administration).
Patients should be advised to notify their healthcare provider if diarrhoea or additional symptoms of colitis occur while taking Piqray. Patients should be managed according to local standard of care medical management, including electrolyte monitoring, administration of anti-emetics and anti-diarrhoeal medications and/or fluid replacement and electrolyte supplements, as clinically indicated. In case of colitis, additional treatment, such as steroids, may be considered as clinically indicated.
Use in hepatic impairment.
See Section 4.2 Dose and Method of Administration, Special populations; Section 5.2 Pharmacokinetic Properties.
Use in renal impairment.
See Section 4.2 Dose and Method of Administration, Special populations; Section 5.2 Pharmacokinetic Properties.
Use in the elderly.
See Section 4.2 Dose and Method of Administration, Special populations; Section 5.2 Pharmacokinetic Properties.
Paediatric use.
See Section 4.2 Dose and Method of Administration, Special populations; Section 5.2 Pharmacokinetic Properties.
Effects on laboratory tests.
See Table 8.4.5 Interactions with Other Medicines and Other Forms of Interactions
The elimination of alpelisib is majorly driven by non-hepatic hydrolysis, mediated by multiple enzymes (esterases, amidases, choline esterase) and to a lesser degree, CYP3A4 mediated metabolism (hydroxylation). The contribution of hepatobiliary export or intestinal secretion via breast cancer resistance protein (BCRP) in human is considered to be low.
Medicinal products that may increase alpelisib plasma concentrations.
BCRP inhibitors.
Alpelisib is a sensitive substrate for BCRP in vitro, predominantly expressed in the liver, intestine, and at blood-brain barrier. Absorption of alpelisib will not be affected by BCRP inhibition due to saturation of the transporter in the intestine. However, due to the involvement of BCRP in the hepatobiliary export and intestinal secretion of alpelisib, caution is advised when co-administering Piqray with a BCRP inhibitor (e.g. eltrombopag, lapatinib, pantoprazole), as inhibition of BCRP in the liver and in the intestine after absorption may lead to an increase in systemic exposure of Piqray.
Medicinal products that may decrease alpelisib plasma concentrations.
CYP3A4 inducers.
Administration of 600 mg once daily rifampin, a strong CYP3A4 inducer, for 7 days, before co-administration with a single oral 300 mg alpelisib dose on Day 8, decreased alpelisib Cmax by 38% and AUC by 57% in healthy adults (N = 25). Administration of 600 mg once daily rifampin for 15 days, co-administered with daily 300 mg alpelisib starting from Day 8 to Day 15 decreased the steady state alpelisib Cmax by 59% and AUC by 74%.
Co-administration with a strong CYP3A4 inducer decreases alpelisib area under the curve (AUC) (see Section 5.2 Pharmacokinetic Properties), which may reduce alpelisib efficacy. Co-administration of alpelisib with strong CYP3A4 inducers (e.g. apalutamide, carbamazepine, enzalutamide, mitotane, phenytoin, rifampin, St. John's wort) should be avoided and selection of an alternative concomitant medicinal product, with no or minimal potential to induce CYP3A4, should be considered (see Section 5.1 Pharmacodynamic Properties).
Medicinal products whose plasma concentrations may be altered by alpelisib.
CYP3A4, CYP2C8, CYP2C9, CYP2C19 and CYP2B6 substrates.
In vitro, alpelisib is a time-dependent inhibitor and an inducer of CYP3A4. No dose adjustment is required when co-administering Piqray with CYP3A4 substrates (e.g. everolimus, midazolam), CYP2C8 substrates (e.g. repaglinide), CYP2C9 substrates (e.g. warfarin) and CYP2C19 substrates (e.g. omeprazole).
In a drug-drug interaction study of healthy subjects, repeated doses of alpelisib 300 mg was co-administered with a single-dose cocktail of sensitive substrates of CYP3A4 (midazolam), CYP2C8 (repaglinide), CYP2C9 (warfarin), CYP2C19 (omeprazole) and CYP2B6 (bupropion). 76.5% of patients were white, and the majority of subjects were intermediate or normal metabolisers for CYP2C9 and CYP2C19. No clinically significant pharmacokinetic interactions were found. The data from CYP2B6 substrate (bupropion) should be interpreted with caution due to the small sample size. S-warfarin exposure increased on average by 34% and 19% for AUCinf and Cmax respectively, compared to administration with S-warfarin alone, which indicates that alpelisib is a mild inhibitor of CYP2C9.
In a drug-drug interaction study in patients with advanced solid tumours, co-administration of alpelisib with everolimus, a sensitive CYP3A4 substrate and a P-gp substrate, showed no clinically significant pharmacokinetic interactions (decrease in AUC by 11.2%). No change in everolimus exposure was observed at alpelisib doses ranging from 250 to 300 mg.
Drug-food interactions.
In healthy subjects, co-administration of alpelisib with food resulted in an increased AUC of alpelisib by 77% (see Section 4.2 Dose and Method of Administration; Section 5.2 Pharmacokinetic Properties). Therefore, Piqray should be taken immediately after food, at approximately same time each day (see Section 4.2 Dose and Method of Administration).
Hormonal contraceptives.
It is currently unknown whether alpelisib may reduce the effectiveness of systemically acting hormonal contraceptives.
Transporter-based interaction.
Alpelisib showed weak in vitro inhibition towards the efflux transporters P-gp, BCRP, and BSEP, solute carrier transporters at the liver inlet (OATP1B1, OATP1B3, and OCT1) and solute carrier transporters in the kidney (OCT2, MATE1, and MATE2K). As unbound systemic steady state concentrations at the therapeutic dose are significantly lower than the experimentally determined unbound inhibition constants or IC50, the inhibition will not translate into clinical significance. However, given the relatively high intestinal and hepatic inlet concentrations of alpelisib, clinically relevant inhibition of oral absorption of P-gp substrates and hepatic uptake of OCT1, OATP1B1 and OAT1B3 substrates may occur. Both alpelisib and the major metabolite, BZG791 inhibited the renal uptake transporter OAT3 (Ki 29.4 and 1.38 microM, respectively) in vitro, and thus alpelisib might increase plasma concentrations of drugs that are predominantly excreted by this transporter. As an in vitro inhibitor of OATP1B1 (IC50 20.9 microM) alpelisib might also increase plasma concentrations of drugs that are OATP1B1 substrates and predominantly cleared by hepatic metabolism.
Fulvestrant.
Data from a clinical study in patients with breast cancer indicated no effect of fulvestrant on alpelisib exposure (and vice versa) following co-administration of the drugs.4.6 Fertility, Pregnancy and Lactation
Effects on fertility.
There are no clinical data available on the effect of Piqray on fertility. Based on findings in animals, Piqray may impair fertility in males and females of reproductive potential.
In repeated-dose toxicity studies up to 13-weeks duration in rats, adverse effects were observed in reproductive organs of females (vaginal epithelial atrophy, vaginal atrophy and oestrus cycle variations) at doses ≥ 2 mg/kg/day (resulting in subclinical exposures) and males (decreased secretion in prostate and seminal vesicle) at doses ≥ 10 mg/kg/day (resulting in subclinical exposure). Prostate glandular atrophy was observed in dogs administered alpelisib at 15 mg/kg/day at 2.3 times the exposure in humans, at the highest recommended human dose of 300 mg based on AUC. In general, the observed effects were reversible or showed a trend towards reversibility after a 4-8 weeks recovery period.
Alpelisib affected male reproductive organs and female fertility in rat fertility and early embryonic development studies. In two fertility studies, male rats were treated for 10 weeks prior to mating (mated with untreated females) and females for 4 weeks prior to mating (mated with untreated males). In females, at doses of 20 mg/kg/day 1.8 times the exposure (based on AUC) in humans at the recommended human dose of 300 mg), increased pre- and post-implantation losses leading to reduced numbers of implantation sites and live embryos. Female fertility was unaffected at 10 mg/kg/day (resulting in subclinical exposures). In males, at doses of ≥ 10 mg/kg/day (exposures approximately equivalent to the clinical AUC), accessory glands weights (seminal vesicles, prostate) were reduced and correlated microscopically with atrophy and/or reduced secretion in prostate and seminal vesicles, respectively. Male fertility parameters were unaffected at doses up to 20 mg/kg/day (1.3 times the exposures in patients at the maximum recommended human dose of 300 mg, based on AUC).
(Category D)
Based on animal data and its mechanism of action, Piqray can cause fetal harm when administered to a pregnant woman.
There are no adequate and well-controlled studies in pregnant women. Embryo-fetal development studies in rats and rabbits have demonstrated that oral administration of alpelisib during organogenesis induced embryo-lethality, feto-toxicity, and teratogenicity. Increased incidences of post-implantation loss, reduced fetal weights, and increased incidences of fetal abnormalities were observed at 10 mg/kg/day in rats and 15 mg/kg/day in rabbits with systemic exposures 0.8 (rat) and 5 times (rabbit) the exposure in humans at the highest recommended dose of 300 mg/day based on AUC.
Piqray should not be used during pregnancy unless the benefits to the mother outweighs the risk to the fetus. If Piqray is used during pregnancy, the patient should be advised of the potential risk to the fetus.
Contraception.
A negative pregnancy status for females of reproductive potential should be verified prior to starting treatment with Piqray.
Females of reproductive potential should be advised that animal studies and the mechanism of action have shown that alpelisib can be harmful to the developing fetus. Sexually active females of reproductive potential should use effective contraception (methods that result in less than 1% pregnancy rates) when using Piqray during treatment and for at least 4 days after stopping treatment with alpelisib. It is currently unknown whether alpelisib may reduce the effectiveness of systemically acting hormonal contraceptives.
Male patients with sexual partners who are pregnant, possibly pregnant, or who could become pregnant should use condoms during sexual intercourse while taking Piqray and for at least 4 days after stopping treatment with Piqray.
It is not known if alpelisib is transferred into human or animal milk after administration of Piqray. There are no data on the effects of alpelisib on the breastfed child or the effects of alpelisib on milk production.
Because of the potential for serious adverse drug reactions in the breastfed child from Piqray, it is recommended that women should not breastfeed during treatment and for at least 4 days after the last dose of Piqray.4.7 Effects on Ability to Drive and Use Machines
Piqray has minor influence on the ability to drive and use machines. Patients should be advised to be cautious when driving or using machines in case they experience fatigue during treatment with Piqray (see Section 4.8 Adverse Effects (Undesirable Effects)).
4.8 Adverse Effects (Undesirable Effects)
Summary of the safety profile.
The overall safety evaluation of Piqray is based on data from the phase III clinical study of 572 patients (571 post-menopausal women and 1 male) who were randomised in a 1:1 ratio to receive Piqray plus fulvestrant or placebo plus fulvestrant; 284 of whom received Piqray at the recommended starting dose of 300 mg dose in combination with fulvestrant, using the proposed treatment regimen.
The median duration of exposure to Piqray plus fulvestrant was 8.2 months with 59.2% patients exposed for > 6 months.
Piqray dose reductions due to adverse events (AEs), regardless of causality occurred in 57.7% of patients receiving Piqray plus fulvestrant and in 4.5% of patients receiving placebo plus fulvestrant. Permanent discontinuations of Piqray and/or fulvestrant due to adverse events were reported in 25% of patients compared to and 4.5% with placebo and/or fulvestrant. The most common AEs leading to treatment discontinuation of both Piqray and/or fulvestrant were hyperglycaemia (6.3%), rash (3.2%), diarrhoea (2.8%), and fatigue (2.1%).
On-treatment deaths, regardless of causality, were reported in 7 patients (2.5%) treated with Piqray plus fulvestrant vs. 12 patients (4.2%) treated with placebo plus fulvestrant. In Piqray plus fulvestrant treated patients, disease progression (5 patients, 1.8%) was the most frequent cause of death; the others were one each for cardio-respiratory arrest and second primary malignancy, neither of which were considered related to treatment with Piqray.
The most common adverse drug reactions (ADRs) in Piqray plus fulvestrant treated patients (reported at a frequency > 20% and for which the frequency for Piqray plus fulvestrant exceeds the frequency for placebo plus fulvestrant) were hyperglycaemia, diarrhoea, rash, nausea, fatigue and asthenia, decreased appetite, stomatitis, vomiting and weight decreased.
The most common Grade 3/4 ADRs (reported at a frequency > 2% in Piqray plus fulvestrant arm and for which the frequency for Piqray plus fulvestrant exceeds the frequency for placebo plus fulvestrant) were hyperglycaemia, rash and rash maculo-papular, fatigue, diarrhoea, lipase increased, hypertension, hypokalaemia, anaemia, weight decreased, gamma-glutamyltransferase increased, lymphopenia, nausea, stomatitis, alanine aminotransferase increased and mucosal inflammation.
Tabulated summary of adverse drug reactions from clinical studies.
ADRs from the phase III clinical study (Table 7) are listed by MedDRA system organ class. Within each system organ class, the ADRs are ranked by frequency, with the most frequent reactions first. Within each frequency grouping, ADRs are presented in order of decreasing seriousness. In addition, the corresponding frequency category for each ADR is based on the following convention (CIOMS III): very common ≥ 1/10, common ≥ 1/100 to < 1/10, uncommon ≥ 1/1,000 to < 1/100, rare ≥ 1/10,000 to < 1/1,000, very rare < 1/10,000.


Adverse drug reactions from spontaneous reports and literature cases (frequency not known).
The following adverse drug reactions have been derived from post-marketing experience with Piqray via spontaneous case reports and literature cases. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency which is therefore categorised as not known. See Table 9.

Description of selected ADRs and treatment recommendations, where applicable.
Hyperglycaemia.
In the phase III clinical study, hyperglycaemia (FPG > 160 mg/dL) was reported in 184 (64.8%) of patients. An event of hyperglycaemia resolved to ≤ Grade 1 (FPG < 160 mg/dL) in 166 (88.8%) of the 187 patients. Dose interruptions and adjustments due to hyperglycaemic events were reported in 26.8% and 28.9% of patients, respectively, in the Piqray plus fulvestrant arm. Hyperglycaemic events leading to discontinuation of Piqray and/or fulvestrant were reported in 19 (6.7%) patients. Based on baseline FPG and HbA1c values, 56% of patients were considered pre-diabetic (FPG > 100-126 mg/dL (5.6 to 6.9 mmol/L) and/or HbA1c 5.7-6.4%) and 4.2% of patients were considered diabetic (FPG ≥ 126 mg/dL (≥ 7.0 mmol/L) and/or HbA1c ≥ 6.5%). There were no patients with type 1 diabetes mellitus based on reported medical history in the phase III clinical study. Among those pre-diabetic patients at baseline, 74.2% experienced hyperglycaemia (any Grade) when treated with Piqray. Among the patients who had Grade ≥ 2 (FPG 160 - 250 mg/dL) hyperglycaemia, the median time to first occurrence of Grade ≥ 2 (FPG > 160 - 250 mg/dL) hyperglycaemia was 15 days (range: 5 days to 517 days) (based on laboratory findings). The median duration of Grade 2 (FPG > 160 - 250 mg/dL) or higher hyperglycaemia (based on laboratory findings) was 10 days (95% CI: 8 to 13 days).
Metformin premedication for hyperglycaemia.
The use of Piqray and endocrine therapy with metformin premedication was evaluated in METALLICA, an open-label, single-arm, two-cohort study in patients with HR-positive, HER2-negative advanced breast cancer harbouring PIK3CA mutation(s). Most patients (93%) received fulvestrant as endocrine therapy during the study. Cohort A enrolled patients with normal glycaemic status (FPG < 100 mg/dL [< 5.6 mmol/L] and HbA1c < 5.7%) and Cohort B enrolled patients with impaired glycaemic status (FPG 100-140 mg/dL [5.6-7.8 mmol/L] or HbA1c 5.7%-6.4%). The overall median age was 55 years.
Metformin was administered beginning 7 days prior to treatment with Piqray. On Day 1 to Day 3, metformin 500 mg twice daily was administered orally and then increased up to 1,000 mg twice daily based on tolerability. All patients received a glucometer to self-monitor blood glucose in addition to laboratory-based monitoring. Concomitant use of systemic glucocorticoids occurred for 37% of patients in METALLICA (in SOLAR-1, this occurred for 43% of patients with a PIK3CA mutation randomised to receive alpelisib + fulvestrant).
Hyperglycaemia adverse reactions occurred in 33% (16/48) and 70% (14/20 patients) in Cohort A and Cohort B, respectively. Grade 3-4 hyperglycaemia occurred in 2.1% (1/48) of patients in Cohort A and 15% (3/20) of patients in Cohort B. The incidence of nausea, vomiting, and diarrhoea adverse reactions, including Grade 3 diarrhoea, increased with metformin premedication. For more information on diarrhoea in patients treated with Piqray, see Section 4.4 Special Warnings and Precautions for Use, Diarrhoea or colitis.
Serious adverse reactions occurred in 22% of patients in the METALLICA study and serious adverse reactions ≥ 2% included diarrhoea (3%), rash (3%) and vomiting (3%).
The most common Grade 3-4 adverse reactions (≥ 5%) were rash (16%), diarrhoea (13%), and hyperglycaemia (6%).
Permanent discontinuation of Piqray due to adverse reactions in the METALLICA study occurred in 19% of patients, and dose modification or interruption of Piqray due to adverse reactions occurred in 56% of patients, of which 28% were dose reductions.
The most common adverse reactions (≥ 30%) in the METALLICA study were diarrhoea (68%), nausea (68%), fatigue (46%), hyperglycaemia (44%), rash (38%), and vomiting (34%).
See Section 4.2 Dose and Method of Administration, Hyperglycaemia, for considerations relating to the use of metformin premedication.
Rash.
In the phase III clinical study, rash events (including rash maculo-papular, rash macular, rash generalised, rash papular, rash pruritic, dermatitis and dermatitis acneiform) were reported in 153 (53.9%) patients. Rash may be accompanied by pruritus and dry skin in some cases. Rash was predominantly mild or moderate (Grade 1 or 2) and responsive to therapy. Maximum Grade 2 and 3 rash events were reported in 13.7% and 20.1% of patients, respectively. There were no Grade 4 cases of rash reported. Among the patients with Grade 2 or 3 rash, the median time to first onset of Grade 2 or 3 rash was 12 days (range: 2 days to 220 days). Dose interruptions and dose adjustments due to rash were reported in 21.8% and 9.2% of patients, respectively, in the Piqray plus fulvestrant arm.
Topical corticosteroid treatment should be initiated at the first signs of rash and systemic corticosteroids should be considered for moderate to severe rashes. Additionally, antihistamines are recommended to manage symptoms of rash. In the Phase III study, among the patients who developed a rash, 73.9% (113/153) reported use of at least one topical corticosteroid and 67.3% (103/153) of at least one oral antihistamine. Systemic corticosteroid were administered for rash events in 23% (66/284) of patients. Of the patients who received systemic corticosteroids, 55% (36/66) received oral corticosteroids for rash. At least one event of rash resolved in the majority of the patients, 141 out of 153 patients (92%). Discontinuation of Piqray and/or fulvestrant treatment due to rash events occurred in 12 patients (4.2%).
A subgroup of 86 patients received anti rash treatment, including anti-histamines, prior to onset of rash. In these patients, rash was reported less frequently than in the overall population, for all Grades rash (26.7% vs 53.9%), Grade 3 rash (11.6% vs 20.1%) and rash leading to permanent discontinuation of Piqray (3.5% vs 4.2%). Accordingly, antihistamines may be initiated prophylactically, at the time of initiation of treatment with Piqray. Based on the severity of rash, Piqray may require dose interruption, reduction, or discontinuation as described in Table 3 Dose modification and management for rash (see Section 4.2 Dose and Method of Administration).
GI toxicity (nausea, diarrhoea, vomiting).
In the phase III study, diarrhoea, nausea and vomiting were reported in 57.7%, 44.7% and 27.1% of the patients, respectively, and led to discontinuation of Piqray and/or fulvestrant in 8 (2.8%), 5 (1.8%) and 3 (1.1%) of the patients, respectively (see Table 7).
Anti-emetics (e.g. ondansetron) and anti-diarrhoeal medications (e.g. loperamide) were used in 27/149 (18.1%) and 104/164 (63.4%) of patients to manage symptoms.
Osteonecrosis of the jaw (ONJ).
In the phase III clinical study, ONJ was reported in 4.2% patients (12/284) in the Piqray plus fulvestrant arm compared to 1.4% patients (4/287) in the placebo plus fulvestrant arm. All patients experiencing ONJ were also exposed to prior or concomitant bisphosphonates (e.g. zoledronic acid) or RANK-ligand inhibitors (e.g. denosumab). Therefore, in patients receiving Piqray and bisphosphonates or RANK-ligand inhibitors, an increased risk of development of ONJ cannot be excluded.
Reporting suspected adverse effects.
Reporting suspected adverse reactions after registration of the medicinal product is important. It allows continued monitoring of the benefit-risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions at www.tga.gov.au/reporting-problems.4.9 Overdose
Symptoms.
There is limited experience of overdose with Piqray in clinical studies. In the clinical studies, Piqray was administered at doses up to 450 mg once daily.
In cases where accidental over-dosage of Piqray was reported in the clinical studies, the adverse events associated with the overdose were consistent with the known safety profile of Piqray and included hyperglycaemia, nausea, asthenia and rash.
Treatment.
General symptomatic and supportive measures should be initiated in all cases of over dosage where necessary. There is no known antidote for Piqray.
For information on the management of overdose, contact the Poisons Information Centre on 131126 (Australia).5 Pharmacological Properties
5.1 Pharmacodynamic Properties
Pharmacotherapeutic group: Antineoplastic agents, other antineoplastic agents.
Anatomical Therapeutic Chemical (ATC Code): L01EM03.
Mechanism of action.
Alpelisib is a class I phosphatidylinositol3kinase (PI3K) inhibitor with higher activity against PI3Kα than other members of class I PI3K. Class I PI3K lipid kinases are key components of the PI3K/AKT/mTOR (mammalian target of rapamycin) signalling pathway.
Gain-of-function mutations in the gene encoding the catalytic α-subunit of PI3K (PIK3CA) lead to activation of PI3Kα manifested by increased lipid kinase activity, growth-factor independent activation of AKT-signalling, cellular transformation and the generation of tumours in preclinical models.
In vitro, alpelisib treatment inhibited the phosphorylation of PI3K downstream targets AKT as well as its various downstream effectors in breast cancer cells and showed activity towards cell lines harbouring a PIK3CA mutation.
In vivo, alpelisib showed good tolerability as well as dose-and time-dependent inhibition of the PI3K/AKT pathway and dose-dependent tumour growth inhibition in relevant tumour xenograft models, including models of breast cancer.
PI3K inhibition by alpelisib treatment has been shown to induce an increase in ER transcription in breast cancer cells, therefore, sensitising these cells to estrogen receptor (ER) inhibition by fulvestrant treatment. Combination of alpelisib and fulvestrant demonstrated increased anti-tumour activity than either treatment alone in xenograft models derived from ER+, PIK3CA mutated breast cancer cell lines (MCF-7 and KPL1).
Pharmacodynamic effects.
In biochemical assays, alpelisib inhibited wild type PIK3α and its 2 most common somatic mutations (H1047R, E545K) (IC50~5 nanomol/L) more potently than the PI3Kδ (IC50 = 60 nanomol/L) and PI3Kγ (IC50 = 560 nanomol/L) isoforms and showed significantly reduced activity against PI3Kβ (IC50 = 1156 nanomol/L).
The potency and selectivity of alpelisib was confirmed at the cellular level in mechanistic and relevant tumour cell lines.
Cardiac electrophysiology.
Serial, triplicate ECGs were collected following a single dose and at steady-state to evaluate the effect of alpelisib on the QTcF interval in patients with advanced cancer. A pharmacokinetic-pharmacodynamic analysis included a total of 134 patients treated with alpelisib at doses ranging from 30 to 450 mg.
The analysis demonstrates the absence of a clinically significant QTcF prolongation at the recommended 300 mg dose with or without fulvestrant. The estimated mean change from baseline in QTcF was < 10 ms (7.2 ms; 90% CI: 5.62, 8.83) at the observed geometric-mean Cmax at steady-state (2900 nanogram/mL) following single agent administration at the recommended 300 mg dose.
Clinical trials.
Placebo-controlled study C2301 - SOLAR-1.
Piqray was evaluated in this pivotal phase III, randomised, double-blind study of Piqray in combination with fulvestrant in men and postmenopausal women with HR+, HER2- locally advanced breast cancer whose disease had progressed or recurred on or after an aromatase inhibitor based treatment (with or without CDK4/6 combination).
A total of 572 patients were enrolled into two cohorts, cohort with PIK3CA mutation or cohort without PIK3CA mutation breast cancer. PIK3CA mutation status was determined by clinical trial assays. There were 341 patients enrolled by tumour tissue in the cohort with a PIK3CA mutation and 231 enrolled in the cohort without a PIK3CA mutation. Of the 341 patients in the cohort with a PIK3CA mutation, 336 (99%) patients had one or more PIK3CA mutations confirmed in tumour tissue using the QIAGEN therascreen PIK3CA RGQ PCR Kit. Out of the 336 patients with PIK3CA mutations confirmed in tumour tissue, 19 patients had no plasma specimen available for testing with the QIAGEN therascreen PIK3CA RGQ PCR Kit. Of the remaining 317 patients with PIK3CA mutations confirmed in tumour tissue, 177 patients (56%) had PIK3CA mutations identified in plasma specimen, and 140 patients (44%) did not have PIK3CA mutations identified in plasma specimen.
Patients were randomised to receive either Piqray 300 mg plus fulvestrant or placebo plus fulvestrant in a 1:1 ratio. Randomisation was stratified by presence of lung and/or liver metastasis and previous treatment with CDK4/6 inhibitor(s).
Within the cohort with a PIK3CA mutation, 169 patients were randomised to receive Piqray in combination with fulvestrant and 172 patients were randomised to placebo in combination with fulvestrant. Within this cohort, 170 (49.9%) patients had liver/lung metastases and 20 (5.9%) patients had received prior CDK4/6 inhibitor treatment.
Within the cohort without PIK3CA mutation, 115 patients were randomised to receive Piqray in combination with fulvestrant and 116 were randomised to receive placebo in combination with fulvestrant. 112 (48.5%) patients had liver/lung metastases and 15 (6.5%) patients had prior CDK4/6 inhibitor treatment.
In the cohort with PIK3CA mutation, 97.7% of patients received prior hormonal therapy and 47.8% of patients had the last setting as metastatic and 51.9% of patients whose last setting was adjuvant therapy. Overall, 85.6% of the patients were considered to have endocrine resistant disease; primary endocrine resistance was observed in 13.2% and secondary endocrine resistance in 72.4% of patients.
In both cohorts with or without PIK3CA mutation, demographics and baseline disease characteristics, ECOG performance status, tumour burden, and prior antineoplastic therapy were well balanced between the study arms.
During the randomised treatment phase, Piqray 300 mg or Piqray matching placebo was administered orally once daily on a continuous basis. Fulvestrant 500 mg was administered intramuscularly on Cycle 1 Day 1 and 15 and then at Day 1 of a 28-day cycle during treatment phase (administration +/- 3 days).
Patients were not allowed to cross over from placebo to Piqray during the study or after disease progression.
The primary end point for the study was progression-free survival (PFS) using Response Evaluation Criteria in Solid Tumours (RECIST v1.1), based on the investigator assessment in patients with a PIK3CA mutation. The key secondary end point was overall survival (OS) for patients with a PIK3CA mutation.
Other secondary endpoints included PFS for patients without a PIK3CA mutation, OS for patients without PIK3CA mutation, as well as overall response rate (ORR) by PIK3CA mutation cohort.
Cohort with PIK3CA mutation. Patients enrolled with a PIK3CA mutation had a median age of 63 years (range 25 to 92). 44.9% patients were 65 years of age or older and < 85 years. The patients included were White (66.3%), Asian (21.7%), Black or African American (1.2%).
Primary analysis.
The study met its primary objective at the final PFS analysis (data cut-off date 12-Jun-2018) demonstrating a statistically significant improvement in PFS by investigator assessment in the PIK3CA mutant cohort for patients receiving Piqray plus fulvestrant, compared to patients receiving placebo plus fulvestrant (hazard ratio [HR] = 0.65 with 95% CI: 0.50, 0.85, one sided stratified log-rank test p = 0.00065), with an estimated 35% risk reduction of disease progression or death in favour of treatment with Piqray plus fulvestrant. The median PFS was prolonged by 5.3 months, from 5.7 months (95% CI: 3.7, 7.4) in the placebo plus fulvestrant arm to 11 months (95% CI: 7.5, 14.5) in the Piqray plus fulvestrant arm.
Primary PFS results were supported by consistent results from a blinded independent review committee (BIRC) assessment in this cohort.
PFS efficacy results from the study are summarised in Table 10 and Figure 1.

 Treatment with the combination of Piqray plus fulvestrant was associated with improvements in ORR relative to placebo + fulvestrant. The ORR was 26.6% (95% CI: 20.1, 34.0) in the Piqray plus fulvestrant arm and 12.8% (95% CI: 8.2, 18.7) in the placebo plus fulvestrant arm.
Treatment with the combination of Piqray plus fulvestrant was associated with improvements in ORR relative to placebo + fulvestrant. The ORR was 26.6% (95% CI: 20.1, 34.0) in the Piqray plus fulvestrant arm and 12.8% (95% CI: 8.2, 18.7) in the placebo plus fulvestrant arm.
For patients with measurable disease at baseline, the ORR was 35.7% (95% CI: 27.4, 44.7) in the Piqray plus fulvestrant arm and 16.2% (95% CI: 10.4, 23.5) in the placebo plus fulvestrant arm.
Final OS analysis.
The final Overall Survival (OS) analysis was conducted using a data cut-off date of 23-Apr-2020 and PFS was re-run using this data cut. With a median duration from randomisation to data cut-off of approximately 42 months, the PFS benefit was sustained and consistent with results from the final PFS analysis.
At the final OS analysis, the study did not meet its key secondary objective. As of the data cut-off date of 23-Apr-2020, a total of 87 (51.5%) deaths were reported in the Piqray plus fulvestrant arm and 94 (54.7%) in the placebo plus fulvestrant arm. The HR was 0.86 (95% CI: 0.64, 1.15; p = 0.15, one-sided) and the pre-specified O'Brien-Fleming efficacy boundary of p ≤ 0.0161 was not crossed.
Median OS was prolonged by 7.9 months, from 31.4 months (95% CI: 26.8, 41.3) in the placebo plus fulvestrant arm to 39.3 months (95% CI: 34.1, 44.9) in the Piqray plus fulvestrant arm. See Figure 2 for details.
 OS subgroup analyses by randomisation stratification factors demonstrated a homogeneous and generally consistent treatment effect per investigator assessment. In patients with prior CDK4/6i treatment (n=20), the median OS in the alpelisib plus fulvestrant arm was 29.8 months (95% CI: 6.7, 38.2) compared to 12.9 months (95% CI: 2.5, 34.6) in the placebo plus fulvestrant arm (HR=0.67; 95% CI: 0.21, 2.18).
OS subgroup analyses by randomisation stratification factors demonstrated a homogeneous and generally consistent treatment effect per investigator assessment. In patients with prior CDK4/6i treatment (n=20), the median OS in the alpelisib plus fulvestrant arm was 29.8 months (95% CI: 6.7, 38.2) compared to 12.9 months (95% CI: 2.5, 34.6) in the placebo plus fulvestrant arm (HR=0.67; 95% CI: 0.21, 2.18).
Cohort without PIK3CA mutation. The proof of concept criteria to conclude a treatment benefit with Piqray and fulvestrant with respect to PFS in patients in the PIK3CA non-mutant cohort were not met (HR = 0.85; 95% CI: 0.58, 1.25) (see Section 4.2 Dose and Method of Administration).
5.2 Pharmacokinetic Properties
The pharmacokinetics (PK) of alpelisib were investigated in patients under an oral dosing regimen ranging from 30 to 450 mg daily. Healthy subjects received single oral doses ranging from 300 mg to 400 mg. The PK was mostly comparable in both oncology patients and healthy subjects.
Absorption.
Following oral administration of alpelisib, median time to reach peak plasma concentration (Tmax) ranged between 2.0 to 4.0 hours, independent of dose, time or regimen. Based on absorption modelling bioavailability was estimated to be very high (> 99%) under fed conditions but lower under fasted conditions (~68.7% at a 300 mg dose). Steady-state plasma levels of alpelisib after daily dosing can be expected to be reached on day 3, following onset of therapy in most patients.
Food effect.
Alpelisib absorption is affected by food. In healthy volunteers after a single 300 mg oral dose of alpelisib, compared to the fasted state, a high-fat high-calorie (HFHC) meal (985 calories with 58.1 g of fat) increased AUCinf by 73% and Cmax by 84%, and a low-fat low-calorie (LFLC) meal (334 calories with 8.7 g of fat) increased AUCinf by 77% and Cmax by 145%. No significant difference was found for AUCinf between LFLC and HFHC with a geometric mean ratio of 0.978 [CI: 0.876, 1.09] showing that neither fat content nor overall caloric intake has a considerable impact on absorption. The increase in gastrointestinal solubility by bile, secreted in response to food intake, is considered to be the driver of the food effect. Hence, Piqray should be taken immediately after food, at approximately same time each day.
Acid reducing agents.
The co-administration of the H2 receptor antagonist ranitidine in combination with a single 300 mg oral dose of alpelisib slightly reduced the bioavailability of alpelisib and decreased overall exposure of alpelisib. In the presence of a LFLC meal, AUCinf was decreased on average by 21% and Cmax by 36% with ranitidine. In the absence of food, the effect was more pronounced with a 30% decrease in AUCinf and a 51% decrease in Cmax with ranitidine compared to the fasted state without co-administration of ranitidine. Piqray can be co-administered with drugs that are acid-reducing agents, if Piqray is taken immediately after food. Population pharmacokinetic analysis showed no significant effect on the PK of Piqray by co-administration of acid reducing agents including proton pump inhibitors, H2 receptor antagonists and antacids.
Distribution.
Alpelisib moderately binds to protein with a free fraction of 10.8% regardless of concentration. Alpelisib was equally distributed between red blood cells and plasma with a mean in vivo blood-to-plasma ratio of 1.03. The volume of distribution of alpelisib at steady-state (Vss/F) is estimated at 114 L (intersubject CV% 49%).
Metabolism.
In vitro studies demonstrated that formation of the hydrolysis metabolite BZG791 by chemical and enzymatic amide hydrolysis was a major metabolic pathway, followed by CYP3A4 mediated hydroxylation. Alpelisib hydrolysis occurs systemically by both chemical decomposition and enzymatic hydrolysis via ubiquitously expressed, high-capacity enzymes (esterases, amidases, and choline esterase) not limited to the liver. CYP3A4-mediated metabolites and glucuronides amounted to ~15% of the dose and BZG791 accounted for ~40-45% of the dose. The rest of the dose, which was found as unchanged alpelisib in urine and faeces, was either excreted as alpelisib or non-absorbed.
Excretion.
Alpelisib exhibits low clearance with 9.2 L/hr (CV% 21%) based on population PK analysis under fed conditions. The population derived half-life, independent of dose and time, was 8 to 9 hours at steady state of 300 mg, once daily.
In human mass-balance study, after oral administration, alpelisib and its metabolites were primarily found in the faeces (81.0%), as alpelisib or metabolised as BZG791. Excretion in the urine is minor (13.5%), with 2% of unchanged alpelisib. Following single oral dose of [14C] alpelisib, 94.5% of the total administered radioactive dose was recovered within 8 days.
Linearity/non-linearity.
The pharmacokinetics were found to be linear with respect to dose and time under fed conditions between 30 and 450 mg. After multiple doses, Alpelisib exposure (AUC) at steady-state is only slightly higher than that of a single dose with an average accumulation of 1.3 to 1.5 with a daily dosing regimen.
Special patient populations.
Renal impairment.
No dose adjustment is necessary in patients with mild or moderate renal impairment. Patients with severe renal impairment have not been studied and caution should be used. Based on a population pharmacokinetic analysis that included 117 patients with normal renal function (eGFR ≥ 90 mL/min/1.73 m2)/ (CLcr ≥ 90 mL/min), 108 patients with mild renal impairment (eGFR 60 to < 90 mL/min/1.73m2)/ (CLcr 60 to < 90 mL/min), and 45 patients with moderate renal impairment (eGFR 30 to < 60 mL/min/1.73 m2), mild and moderate renal impairment had no effect on the exposure of alpelisib (see Section 4.2 Dose and Method of Administration).
Hepatic impairment.
No dose adjustment is necessary in patients with mild, moderate or severe hepatic impairment (Child-Pugh A, B and C).
Based on a pharmacokinetic trial in patients with hepatic impairment, moderate and severe hepatic impairment had negligible effect on the exposure of alpelisib (see Section 4.2 Dose and Method of Administration). The mean exposure for alpelisib was increased by 1.26-fold in patients with severe (GMR: 1.00 for Cmax; 1.26 for AUClast/AUCinf) hepatic impairment.
Based on a population pharmacokinetic analysis that included 230 patients with normal hepatic function, 45 patients with mild hepatic impairment and no patients with moderate hepatic impairment, further supporting the findings from the dedicated hepatic impairment study, mild and moderate hepatic impairment had no effect on the exposure of alpelisib, (see Section 4.2 Dose and Method of Administration).
Paediatric use.
The pharmacokinetics Piqray in paediatric patients have not been established.
Use in the elderly.
Of 284 patients who received Piqray in the phase III study (in Piqray plus fulvestrant arm), 117 patients were ≥ 65 years of age and 34 patients were ≥ 75 years of age. No overall differences in safety or effectiveness of Piqray were observed between these patients and younger patients (see Section 4.2 Dose and Method of Administration).
Age, body weight, and gender.
The population PK analysis showed that there are no clinically relevant effects of age, body weight, or gender on the systemic exposure of alpelisib that would require Piqray dose adjustment.
Race/ethnicity.
Population PK analyses and PK analysis from a single agent study in Japanese cancer patients showed that there are no clinically relevant effects of ethnicity on the systemic exposure of Piqray.
Non-compartmental PK parameters after single and multiple daily doses of Piqray for Japanese patients were very similar to those reported in the Caucasian population.
5.3 Preclinical Safety Data
Cardiovascular safety pharmacology.
In an in vitro hERG test, (where functionality of the human cardiac hERG channel heterologously expressed in HEK293 cells in vitro is assessed), an IC50 of 9.4 microM (4.2 microgram/mL) was found. No relevant electrophysiological effect was seen in dogs in several studies, up to single doses of 180 mg/kg in vivo. An in vivo telemetry study in dogs showed an elevated blood pressure, starting at exposure lower than the exposure in humans, at the highest recommended dose of 300 mg/day.
Genotoxicity.
Alpelisib was not mutagenic in an in vitro bacterial reverse mutation (Ames) assay, or aneugenic or clastogenic in human cell micronucleus and chromosome aberration tests in vitro. Alpelisib was not genotoxic in an in vivo rat micronucleus test.
Carcinogenicity.
Carcinogenicity studies have not been conducted with alpelisib.6 Pharmaceutical Particulars
6.1 List of Excipients
Piqray tablets contain the following inactive ingredients: microcrystalline cellulose, mannitol, sodium starch glycollate, hypromellose, magnesium stearate (vegetable source), macrogol, iron oxide red CI77491, iron oxide black CI77499, titanium dioxide (E171), and purified talc.
6.2 Incompatibilities
Incompatibilities were either not assessed or not identified as part of the registration of this medicine.
6.3 Shelf Life
In Australia, information on the shelf life can be found on the public summary of the Australian Register of Therapeutic Goods (ARTG). The expiry date can be found on the packaging.
6.4 Special Precautions for Storage
Store in the original package in order to protect from moisture. Keep out of reach of children.
6.5 Nature and Contents of Container
Piqray tablets are supplied in PVC/PCTFE (polyvinylchloride/polychlorotrifluoroethylene) or PA/Al/PVC (polyamide/aluminium/polyvinylchloride)/aluminium laminate blister packs.
Piqray 50 mg and Piqray 200 mg film-coated tablets.
14 day* or 28 day calendar packs containing 28 film-coated tablets (fourteen 50 mg and fourteen 200 mg) or 56 film-coated tablets (twenty-eight 50 mg and twenty-eight 200 mg).
Piqray 150 mg film-coated tablets.
14 day* or 28 day calendar packs containing 28 or 56 film-coated tablets.
Piqray 200 mg film-coated tablets.
14 day* or 28 day calendar packs containing 14 or 28 film-coated tablets.
*Not all pack sizes are supplied.
6.6 Special Precautions for Disposal
Any unused product should not be disposed of in household waste or wastewater. Return it to a pharmacist for safe disposal.
6.7 Physicochemical Properties
Chemical name (IUPAC): (2S)-N1-{4-Methyl-5-[2-(1,1,1-trifluro-2-methylpropan-2-yl) pyridin- 4-yl]-1,3-thiazol-2-yl} pyrrolidine-1,2-dicarboxamide.
Molecular formula: C19H22F3N5O2S.
Relative molecular mass: 441.47.
Alpelisib is a white to almost white powder at room temperature. It is sparingly soluble in methanol, acetone, and absolute ethanol; it is insoluble/practically insoluble in water, and demonstrates pH-dependent aqueous solubility. Alpelisib is most soluble at pH 1 and has two experimentally determined dissociation constants with pKa values of 3.3 and 9.4. The pH of a 1.0% (m/v) solution of alpelisib in water/ethanol (50:50 v/v) is approximately 6.2. In an n-octanol/pH 6.8 buffer system, alpelisib has a log D of 2.8. Alpelisib is optically active.
Chemical structure.

CAS number.
Chemical Abstracts Service (CAS) number: 1217486-61-7.7 Medicine Schedule (Poisons Standard)
Schedule 4 - Prescription Only Medicine.
Summary Table of Changes



 Tables 2, 3, 4 and 5 summarise recommendations for dosing interruption, reduction or discontinuation of Piqray in the management of specific ADRs. Clinical judgment of the treating physician, including confirmation of laboratory values if deemed necessary, should guide the management plan of each patient based on the individual benefit/risk assessment for treatment with Piqray.
Tables 2, 3, 4 and 5 summarise recommendations for dosing interruption, reduction or discontinuation of Piqray in the management of specific ADRs. Clinical judgment of the treating physician, including confirmation of laboratory values if deemed necessary, should guide the management plan of each patient based on the individual benefit/risk assessment for treatment with Piqray.



 In the phase III clinical study, in patients with hyperglycaemia, 163/187 (87.2%) were managed with anti-diabetic medication and 142/187 (75.9%) reported use of metformin as single agent or in combination with other anti-diabetic medication. The maximum dose of metformin recommended in phase III clinical study was 2000 mg per day.
In the phase III clinical study, in patients with hyperglycaemia, 163/187 (87.2%) were managed with anti-diabetic medication and 142/187 (75.9%) reported use of metformin as single agent or in combination with other anti-diabetic medication. The maximum dose of metformin recommended in phase III clinical study was 2000 mg per day.



 Treatment with the combination of Piqray plus fulvestrant was associated with improvements in ORR relative to placebo + fulvestrant. The ORR was 26.6% (95% CI: 20.1, 34.0) in the Piqray plus fulvestrant arm and 12.8% (95% CI: 8.2, 18.7) in the placebo plus fulvestrant arm.
Treatment with the combination of Piqray plus fulvestrant was associated with improvements in ORR relative to placebo + fulvestrant. The ORR was 26.6% (95% CI: 20.1, 34.0) in the Piqray plus fulvestrant arm and 12.8% (95% CI: 8.2, 18.7) in the placebo plus fulvestrant arm. OS subgroup analyses by randomisation stratification factors demonstrated a homogeneous and generally consistent treatment effect per investigator assessment. In patients with prior CDK4/6i treatment (n=20), the median OS in the alpelisib plus fulvestrant arm was 29.8 months (95% CI: 6.7, 38.2) compared to 12.9 months (95% CI: 2.5, 34.6) in the placebo plus fulvestrant arm (HR=0.67; 95% CI: 0.21, 2.18).
OS subgroup analyses by randomisation stratification factors demonstrated a homogeneous and generally consistent treatment effect per investigator assessment. In patients with prior CDK4/6i treatment (n=20), the median OS in the alpelisib plus fulvestrant arm was 29.8 months (95% CI: 6.7, 38.2) compared to 12.9 months (95% CI: 2.5, 34.6) in the placebo plus fulvestrant arm (HR=0.67; 95% CI: 0.21, 2.18).
