What is in this leaflet
This leaflet answers some common questions about PLACIL.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have benefits and risks. Your doctor has weighed the risks of you taking PLACIL against the benefits expected for you.
If you have any concerns about taking this medicine, talk to your doctor or pharmacist.
Please read this leaflet carefully and keep it with your medicine. You may need to read it again.
What PLACIL is used for
PLACIL is used to treat:
- depression
- obsessive-compulsive disorders and phobias in adults
- muscle weakness in people with a sleep disorder called narcolepsy.
Depression is thought to be caused by a chemical imbalance in parts of the brain. This imbalance affects your whole body and can cause emotional and physical symptoms such as feeling low in spirit, loss of interest in activities, being unable to enjoy life, poor appetite or overeating, disturbed sleep, loss of sex drive, lack of energy and feelings of guilt.
PLACIL belongs to a group of medicines called tricyclic antidepressants. These medicines are thought to work by their action on brain chemicals called amines.
Ask your doctor if you have any questions about why PLACIL has been prescribed for you. Your doctor may have prescribed PLACIL for another reason.
PLACIL is not approved for use in children and adolescents below 18 years of age for the treatment of depression and other psychiatric disorders. The safe use and effectiveness of PLACIL in treating the above conditions, for this age group, has not been established.
PLACIL is available only with a doctor's prescription.
Before you take PLACIL
When you must not take it
Do not take PLACIL if you are allergic to:
- medicines containing clomipramine or any other tricyclic antidepressant
- any of the ingredients listed at the end of this leaflet.
Symptoms of an allergic reaction may include skin rash, itching or hives; swelling of the face, lips or tongue, difficulty in swallowing; wheezing or shortness of breath.
Do not take PLACIL if you are taking, or have taken within the last 14 days, another medicine for depression called a monoamine oxidase inhibitor (MAOI). Taking PLACIL together with a MAOI or taking it too soon after stopping a MAOI may cause a serious reaction with a sudden increase in body temperature, extremely high blood pressure and seizures (fits). Your doctor will tell you when it is safe to start taking PLACIL after stopping the MAOI.
Ask your doctor or pharmacist if you are not sure if you have been taking one of these medicines.
Do not take PLACIL if you have:
- recently had a heart attack
- congenital long QT syndrome - people with this inherited heart condition have electrical disturbances in the heart, which may cause sudden, extremely rapid heart rates.
Taking PLACIL could make your condition worse.
Do not take PLACIL if the expiry date (EXP) printed on the pack has passed. If you take this medicine after the expiry date has passed, it may not work as well.
Do not take PLACIL if the packaging shows signs of tampering or if the tablets do not look right.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you are allergic to any other medicines, foods, dyes or preservatives.
Tell your doctor if you are pregnant or plan to become pregnant. There have been reports of some babies experiencing complications immediately after delivery. Your doctor will discuss the possible risks and benefits of taking PLACIL during pregnancy.
Tell your doctor if you smoke. Nicotine in cigarette smoke can affect the amount of PLACIL that is in your body. Changes in your usual smoking habits can also change the effects of PLACIL.
Tell your doctor if you are breastfeeding or wish to breastfeed. Breastfeeding is not recommended while taking PLACIL. The active ingredient passes into breast milk and may affect your baby.
Tell your doctor if you have, or have had, any medical conditions, especially the following:
- heart or blood vessel problems including coronary heart disease, angina (chest pain), abnormal heart beat, or congenital long QT syndrome
- liver or kidney problems
- high or low blood pressure
- glaucoma, a condition characterised by an increased pressure in the eye
- difficulty in passing urine, due to prostate problems or any other cause
- overactive thyroid
- any mental illness other than the one being treated (e.g. mania, bipolar disorders, schizophrenia)
- Parkinson's disease
- epilepsy or (seizures) fits
- tumour of the adrenal gland
- chronic constipation
- hypokalaemia, or low potassium levels in the blood
- lactose or galactose intolerance
(PLACIL tablets contain sugars as lactose monohydrate).
If you have not told your doctor about any of the above, tell them before you start PLACIL.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you buy without a prescription from a pharmacy, supermarket or health food shop.
Some medicines may be affected by PLACIL, or may affect how well it works. These include:
- other medicines for depression such as:
- MAOIs, such as phenelzine (e.g. Nardil), tranylcypromine (e.g. Parnate) and moclobemide (e.g. Aurorix, Arima) (see "When you must not take it" section)
- SSRIs and SNRIs, such as fluoxetine (e.g. Prozac, Lovan), paroxetine (e.g. Aropax, Paxtine), sertraline (e.g. Zoloft), fluvoxamine (e.g. Luvox, Movox), and nefazodone (Serzone) - sleeping tablets or sedatives
- some medicines for anxiety
- other medicines for mental illness
- some medicines for high blood pressure or irregular heart beats
- certain cough and cold preparations including nose drops
- antihistamines, medicines for allergy, hayfever or travel sickness
- medicines to relieve gut cramps or spasms, e.g. hyoscine (Buscopan), mebeverine (Colofac)
- certain medicines for Parkinson's disease such as biperiden (Akineton), benztropine (Cogentin)
- medicines for epilepsy, such as carbamazepine (Teril, Tegretol) or phenytoin (Dilantin)
- medicines to prevent blood clots, such as warfarin (Coumadin, Marevan)
- cimetidine (Tagamet, Magicul), a medicine used to treat reflux and ulcers
- methylphenidate (Ritalin, Attenta), a medicine used to treat attention deficit hyperactivity disorder (ADHD) and narcolepsy
- medicines containing estrogen such as birth control pills and hormone replacement therapy
- nicotine in medicines used to help you quit smoking, such as nicotine gum, patches, lozenges or inhalers
- diuretics, also called fluid tablets
- disulfiram, a medicine used to deter alcohol consumption
- medicines for thyroid problems
- rifampicin, an antibiotic.
- terbinafine, a medicine used to treat skin, hair or nail infections due to fungus
- medicines used to reduce fat in blood
- grapefruit/grapefruit juice or cranberry juice.
Your doctor can tell you what to do if you are taking any of these medicines.
If you are not sure whether you are taking any of these medicines, check with your doctor or pharmacist. Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking PLACIL.
How to take PLACIL
Follow all directions given to you by your doctor and pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the pack, ask your doctor or pharmacist.
How much to take
The dose varies from person to person.
Take PLACIL exactly as directed by your doctor. Your doctor will tell you how many tablets you need to take each day and when to take them. The dose may depend on your age, your condition and whether or not you are taking any other medicines.
PLACIL is usually started using low doses. Your doctor may gradually increase this dose depending on how you respond to this medicine.
For depression, obsessive compulsive disorders and phobias, treatment is usually started with a low dose of 2 or 3 tablets (50 to 75 mg) each day. The dose can be increased slowly up to 4 to 6 tablets (100 to 150 mg) each day. Some people will need higher doses than others because each person's body chemistry is different. Once you are feeling better, your doctor may be able to slowly reduce the dose. The usual adult maintenance dose is between 2 to 4 tablets (50 to 100 mg) each day.
For muscle weakness in people with narcolepsy, the dose is usually from 1 to 3 tablets (25 to 75 mg) each day.
If you are older than 65 years old, your doctor will probably start with a low dose (e.g 1 tablet a day (25 mg)) to help avoid side effects. The dose is gradually increased over about ten days to 2 to 3 tablets (50 to 75 mg) each day and kept at that dose for the rest of your treatment.
How to take it
Swallow the tablets with a glass of water.
PLACIL can be taken with or without food.
If your stomach is upset after taking the tablets, take them with a meal or after a snack.
When to take it
The tablets are usually taken in 2 to 3 doses spaced throughout the day, unless your doctor advises you otherwise.
Take your medicine at about the same time each day. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
If you have narcolepsy and you have trouble sleeping at night, take the last dose before the evening to avoid making your insomnia worse.
If you forget to take it
If it is almost time for your next dose (e.g. within 2 or 3 hours), skip the dose you missed and take the next one when you are meant to.
Otherwise, take the dose as soon as you remember, and then go back to taking the tablets as you would normally.
Do not take a double dose to make up for the dose you missed. This may increase the chance of you getting an unwanted side effect.
If you have trouble remembering when to take your medicine, ask your pharmacist for some hints.
If you are not sure what to do, check with your doctor or pharmacist.
How long to take it
Keep taking PLACIL for as long as your doctor recommends. The length of treatment will depend on your condition and how you respond to PLACIL.
Most medicines of this type take time to work, so do not be discouraged if you do not feel better right away.
Some of your symptoms may improve in 1 or 2 weeks, but it can take up to 4 or 6 weeks to feel the full benefit of PLACIL. Even when you feel well, you will usually have to take PLACIL for several months or longer, to make sure that the benefits last.
If you take too much (overdose)
Immediately telephone your doctor, or the Poisons Information Centre (telephone 13 11 26), or go to Accident and Emergency at the nearest hospital; if you think you or anyone else may have taken too much PLACIL.
Do this even if there are no signs of discomfort or poisoning.
Keep the telephone numbers for these places handy. You may need urgent medical attention.
If you take too much PLACIL, you may feel sleepy, restless or agitated, stiff or have unusual muscle movements. You may also vomit, sweat, have a fever, fits, have an irregular heartbeat, drop in blood pressure or have difficulty breathing. You may lose consciousness.
Keep PLACIL out of the reach of children. Children are much more sensitive than adults to tricyclic antidepressants such as PLACIL. An accidental overdose is especially dangerous.
While you are taking PLACIL
Things you must do
Tell your doctor immediately if you have any suicidal thoughts or other mental/mood changes. Occasionally, the symptoms of depression or other psychiatric conditions may include thoughts of harming yourself or committing suicide. These symptoms may continue or get worse during the first one to two months of treatment until the full antidepressant effect of the medicine becomes apparent. This is more likely to occur in young adults under 25 years of age.
Contact your doctor or a mental health professional right away or go to the nearest hospital for treatment if you or someone you know is showing any of the following warning signs of suicide:
- new or worsening depression
- new or worsening anxiety
- feeling very agitated or restless
- panic attacks
- thoughts or talk of death or suicide
- thoughts or talk of self-harm or harm to others
- any recent attempts of self-harm or suicide
- increase in aggressive behaviour, irritability or any other unusual changes in behaviour or mood.
- new or worsening irritability
- difficulty sleeping (insomnia)
- acting on dangerous impulses
- an extreme increase in activity and talking.
All mentions of suicide or violence must be taken seriously.
Keep all of your appointments with your doctor so that your progress can be checked. Your doctor may want to take some blood tests and check your heart and blood pressure from time to time. This helps prevent unwanted side effects.
Tell your doctor immediately if you become pregnant while taking PLACIL. Do not stop taking your tablets until you have spoken to your doctor.
Before starting any new medicine, tell your doctor or pharmacist that you are taking PLACIL.
Tell all the doctors, dentists and pharmacists who are treating you that you are taking PLACIL.
If you plan to have any surgery, including dental surgery or minor surgery, tell your doctor, anaesthetist or dentist that you are taking PLACIL.
Your doctor may ask you to stop taking PLACIL a few days before elective surgery. Tell your doctor if, for any reason, you have not taken your medicine exactly as prescribed. Otherwise, your doctor may think that it was not effective and change your treatment unnecessarily.
Tell your doctor if you feel the tablets are not helping your condition.
Things you must not do
Do not stop taking PLACIL or lower the dose without checking with your doctor. Do not let yourself run out of medicine over weekends or holidays. Suddenly stopping PLACIL may cause nervousness, vomiting, diarrhoea, headache and nausea. It can also worsen your condition.
Your doctor will tell you how to gradually reduce the amount of PLACIL you are taking before stopping completely.
Do not use PLACIL to treat any other conditions unless your doctor tells you to.
Do not give PLACIL to anyone else, even if they have the same condition as you.
Things to be careful of
Be careful driving or operating machinery until you know how PLACIL affects you. PLACIL may cause drowsiness, blurred vision, dizziness or lightheadness in some people. If any of these occur, do not drive, operate machinery or do anything else that could be dangerous.
Be careful when drinking alcohol or taking pain relievers, sleeping tablets or antihistamines (medicines for colds or allergies such as hay fever) while you are taking PLACIL. Combining alcohol with PLACIL can make you more drowsy, dizzy or lightheaded. Your doctor may suggest you avoid alcohol while being treated with PLACIL.
Be careful getting up from a sitting or lying position. Dizziness, lightheadedness or fainting may occur, especially when you get up quickly. Getting up slowly may help.
You can usually prevent these symptoms by getting up slowly and flexing leg muscles and toes to get the blood flowing. When getting out of bed, dangle your legs over the side for a minute or two before standing up.
Be careful to stay out of direct sunlight as much as possible until you find out if your skin is more sensitive than usual. PLACIL may cause your skin to be more sensitive than usual to sunlight. Wear protective clothing and use SPF 30+ sunscreen.
If you wear contact lenses and find that your eyes are dry, sticky or irritated, tell your doctor. These side effects could damage your eyes.
Tell your doctor or dentist if your mouth continues to feel dry for more than 2 weeks. PLACIL may cause dry mouth. This can be relieved by frequent sips of water, sucking sugarless lollies or chewing sugarless gum. However, continuing dryness of the mouth may increase the chance of dental disease, including tooth decay and gum disease.
Families and carers should be aware that special care might be needed when elderly patients take PLACIL. They may become confused and are more likely to experience side effects when taking PLACIL.
After you have stopped taking PLACIL, you should still be careful for 1 or 2 weeks since some of the effects of the medicine will still be in your body.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking PLACIL. Like all other medicines, PLACIL may have unwanted side effects in some people. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
As people grow older, they are more likely to get side effects from medicines.
If you are over 65 years of age, you may have an increased chance of getting side effects. Report any side effects promptly to your doctor. PLACIL can cause confusion or disorientation, especially in older people or those with Parkinson's disease. Your family or carer may be needed.
Patients aged 50 years or older and taking a medicine from this group are more likely to experience bone fractures.
Ask your doctor or pharmacist any questions you may have.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Tell your doctor if you notice any of the following and they worry you:
- dryness of the mouth
- sweating, hot flushes
- feeling sick (nausea), vomiting, diarrhoea
- changes in taste
- change in appetite, weight gain or weight loss
- constipation
- blurred vision, difficulty focussing eyes, especially when treatment is started or dose is increased.
- Lightheadedness, especially when you get up too quickly from a sitting or lying down position.
- dizziness, shakiness, muscle spasms
- drowsiness, tiredness
- headache, loss of memory or reduced concentration, mental dullness
- restlessness, anxiety
- confusion, hallucinations
- difficulty in passing urine or frequent passing of large amounts of urine
- changes in sex drive, or difficulty reaching orgasm or delayed or no ejaculation of semen if you are a male
- ringing in the ears
- increased sensitivity to the sun
- change in sense of taste.
- dry or sticky eyes if you wear contact lenses
- compelling need to be in constant motion
- repetitive, involuntary, purposeless movements
- disturbed sleep or nightmares
- sores in the mouth or tongue
- swelling of the breast or discharge of milk
- swelling of testicles
- hair loss
Tell your doctor immediately if you notice any of the following:
- any numbness, weakness or tingling of the arms or legs
- unsteadiness when walking or difficulty coordinating your movements
- weakness or loss of balance
- difficulty in speaking or slurred speech
- severe drowsiness or dizziness.
- signs of frequent infections such as fever, chills, sore throat or swollen glands (constant flu-like symptoms)
- bruising or bleeding more easily than usual
- eye pain
- signs of liver disease such as nausea, vomiting, loss of appetite, feeling generally unwell, fever, itching, yellowing of the skin and eyes, and dark coloured urine
- abnormal ideas or hallucinations
- mood swings alternating from one of excitement, overactivity and uninhibited behaviour to a depressed mood.
- pain in the stomach or abdomen that is severe or doesn't go away.
The above list includes serious side effects that require medical attention.
See your doctor immediately or go to Accident and Emergency at the nearest hospital if you notice any of the following:
- fainting, collapse
- fast, irregular or pounding heart beat, chest pain
- fits or seizures
- a sudden increase in body temperature, extremely high blood pressure and severe convulsions
- signs of an allergic reaction such as skin rash, itching or hives, swelling of the face, lips or tongue, difficulty in swallowing, wheezing or shortness of breath.
- symptoms like agitation, confusion, diarrhoea, high temperature, increased blood pressure, excessive sweating and rapid heartbeat (a syndrome due to an increase in naturally occurring messenger, serotonin)
These are very serious side effects. You may need urgent medical attention or hospitalisation.
Tell your doctor if you notice anything that is making you feel unwell.
This is not a complete list of all possible side effects.
Other side effects not listed above may also occur in some patients.
After using PLACIL
Storage
Keep PLACIL where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Keep your tablets in a cool dry place where the temperature stays below 25°C.
Do not store PLACIL or any other medicine in the bathroom or near a sink.
Do not leave PLACIL in the car or on windowsills. Heat and dampness can destroy some medicines.
Disposal
If your doctor tells you to stop taking PLACIL, or your tablets have passed their expiry date, ask your pharmacist what to do with any that are left over.
Product description
What it looks like
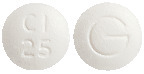
PLACIL is a white, biconvex tablet, marked "Cl 25" on one side and "G" on the other.
Each pack contains 50 tablets.
Ingredients
The active ingredient in PLACIL is clomipramine hydrochloride. Each PLACIL tablet contains 25 mg of clomipramine hydrochloride.
The tablets also contain:
- lactose monohydrate
- maize starch
- povidone
- sodium starch glycollate
- magnesium stearate
- purified talc
- Instacoat Universal White (A05G15138) ARTG PI No: 144681.
PLACIL tablets contain sugar as lactose and trace quantities of sulfites.
Sponsor
Alphapharm Pty Ltd trading as Viatris
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.viatris.com.au
Phone: 1800 274 276
Australian registration number:
AUST R 143879
This leaflet was prepared on
May 2023.
PLACIL® is a Viatris company trade mark
PLACIL_cmi\May23/00
Published by MIMS June 2023

 Chemical name: 3-chloro-5-[3-(dimethylamino)-propyl]-10,11-dihydro-5H-dibenz[b,f]azepine hydrochloride.
Chemical name: 3-chloro-5-[3-(dimethylamino)-propyl]-10,11-dihydro-5H-dibenz[b,f]azepine hydrochloride.