What is in this leaflet
This leaflet answers some common questions about Plaquenil Tablets. It does not contain all the available information.
It does not take the place of talking to your doctor or pharmacist.
All medicines have benefits and risks. In deciding to give you Plaquenil, your doctor has weighed the risks of taking Plaquenil against the benefits it will have for you.
Keep this information with the tablets. You may wish to read it again later.
What is Plaquenil used for
Plaquenil may be used for any of the following conditions:
Rheumatoid arthritis
Rheumatoid arthritis is a form of arthritis with inflammation of the joints, characterised by stiffness, swelling and pain. Plaquenil may be used for short or long-term rheumatoid arthritis treatment.
In treating rheumatoid arthritis, Plaquenil may slow down the process of joint damage and relieve the symptoms of the disease.
Systemic Lupus Erythematous (SLE)
SLE is a disease in which a person's normal immunity is upset. The body produces an excess of blood proteins called antibodies and these antibodies may cause problems in any organ of the body.
These antibodies may end up, for example, in the skin causing a variety of skin rashes or deposit in the kidney, brain, lung and joints causing injury.
Discoid Lupus Erythematous (DLE)
DLE is similar to SLE except it only affects the skin and is characterised by a scaling, red rash.
Malaria (treatment and control of symptoms)
Malaria is an infectious disease caused by the presence of parasites in red blood cells.
The disease is characterised by chills, fever and sweats.
In malaria, Plaquenil destroys the harmful parasite which causes the illness.
Your doctor may have prescribed this medicine for another reason. Ask your doctor if you have any questions about why Plaquenil has been prescribed for you.
Plaquenil is not addictive. This medicine is available only with a doctor's prescription.
Before you take Plaquenil
When you must not take Plaquenil
Do not take Plaquenil if you have ever had an allergic reaction to hydroxychloroquine, chloroquine, or related products or any of the ingredients listed under "Product Description".
If you are uncertain whether you have had an allergic reaction to a related product ask your doctor or pharmacist.
The symptoms of an allergic reaction may include an asthma attack, facial swelling, skin rash or hay fever.
Ask your doctor about the risks and benefits of taking Plaquenil while you are pregnant. When Plaquenil is taken for long periods of time, there is an increased risk to the unborn child. It may cause problems with brain function, hearing, balance and vision.
Ask your doctor about the risks and benefits of taking Plaquenil while you are breastfeeding.
Do not take Plaquenil if you have previously experienced changes in your eyesight when taking medicines for rheumatoid arthritis or malaria.
Plaquenil should not be used in children under 6 years.
Plaquenil should not be used in children over 6 years for long periods.
Do not take Plaquenil after the expiry date printed on the bottle. It may have no effect at all, or worse, an entirely unexpected effect if you take it after the expiry date.
Do not take Plaquenil if the bottle is damaged or shows signs of tampering.
Do not take Plaquenil to treat any other complaint unless your doctor says it is safe. Do not give this medicine to anyone else.
Before you start to take Plaquenil
You must tell your doctor if:
- You are taking any other medicines for any medical condition
- You are allergic to quinine.
- You have allergies to any ingredients listed under "Product Description" at the end of this leaflet.
- You have any pre-existing eye disorders.
- You have experienced low blood sugar levels (hypoglycaemia - a "hypo"). Plaquenil may increase the risk of you having a hypo.
- You have or have had any of these medical conditions:
- Chloroquine-resistant malaria
- Liver or kidney problems
- Diabetes
- Stomach, brain or blood disorders
- Disease of the heart muscle
- Skin diseases, in particular psoriasis which is a kind of itchy rash.
- Glucose-6-phosphate dehydrogenase (G-6-PD) deficiency which is a lack of a chemical substance which causes the breakdown of sugar in the body.
- Porphyria, which is a rare disease of blood pigments.
If you have not told your doctor about any of these things, tell him/her before you take any Plaquenil.
Taking Plaquenil with other medicines
Tell your doctor if you are taking any other medicines, including any that you buy without a prescription from your pharmacy, supermarket or health food shop. Some medicines may interfere with Plaquenil. These include:
- Any medicine to treat depression, including the herbal product St John's wort
- Digoxin - a medicine used to treat heart disease
- Medicines to treat diabetes
- Medicines used to suppress the immune system such as ciclosporin
- Antiarrythmic drugs such as amiodarone and moxifloxacin
- Other antimalarial drugs
- Medicines to treat epilepsy, such as carbamazepine and phenobarbital
- Tamoxifen (a medicine used to treat breast cancer)
- Anti-infective medicines
- Medicines that may affect your blood
- Medicines that may affect your eyes
- Antacids containing magnesium or kaolin or cimetidine, used to neutralise stomach acid
- Itraconazole, an antifungal medication
- Clarithromycin and rifampicin (antibiotics)
- Grapefruit juice
- Anticoagulant drugs such as dabigatran and clopidogrel
- Medicines to treat high cholesterol, such as gemfibrozil
- Ritonavir (a medicine used to treat HIV)
These medicines may be affected by Plaquenil or affect the way Plaquenil works.
Your doctor or pharmacist can tell you what to do if you are taking any of these medicines.
How to take Plaquenil
Swallow tablets whole with a little water or other liquid.
It is best to take Plaquenil at meal times.
The dosage will depend on why you are being treated with Plaquenil.
The usual doses are:
Rheumatoid arthritis
Adults
2-3 tablets daily. Your doctor may later reduce this to 1-2 tablets daily.
SLE and DLE
Adults
2-4 tablets daily. Your doctor may later reduce this to 1-2 tablets daily.
Control of Malaria Symptoms
Adults
2 tablets once a week. The tablets should be taken on exactly the same day of each week.
For example, if your first dose is taken on a Monday, then each weekly dose should be taken on a Monday.
Treatment of malaria
Adults
The starting dose is 4 tablets. Take another 2 tablets six to eight hours later and two further tablets on each of the next 2 days.
Always follow the instructions given to you by your doctor.
Dosages for children are calculated according to the child's body weight.
Your doctor will work out the correct dose for you.
Plaquenil should not be used in children for long periods.
Your doctor may ask you to take a different dose. You should follow the instructions on the label.
If you are unsure what dose to take ask your pharmacist or doctor.
If you forget to take Plaquenil
If you are being given Plaquenil for rheumatoid arthritis, SLE or DLE, do not take a double dose to make up for the dose missed. Just continue with the appropriate dose on the next day.
If you are being given Plaquenil for suppression or treatment of malaria, you should take your tablets as soon as you remember, and go back to taking it as you would normally.
If you have trouble remembering when to take your medicine, ask your pharmacist for some hints.
If you take too much Plaquenil (Overdose)
Immediately telephone your doctor, or the Poisons Information Centre (in Australia telephone 13 11 26 and in New Zealand telephone 0800 POISON or 0800 764766), or go to Accident and Emergency at your nearest hospital, if you think that you or anyone else may have taken too much Plaquenil.
Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
If you take too many tablets you may experience headaches, drowsiness, visual disturbances or fits.
These symptoms may occur within 30 minutes of overdose.
While you are taking Plaquenil
If you are about to start taking any new medicines, tell your doctor and pharmacist that you are taking Plaquenil.
Tell all doctors, dentists and pharmacists who are treating you that you are taking Plaquenil.
Tell your doctor if you experience any of the following symptoms including; weakness, trembling or shaking, sweating, light-headedness, headache, dizziness, lack of concentration, tearfulness or crying, irritability, hunger and numbness around the lips and fingers.
These symptoms may be associated with hypoglycaemia.
If you experience any of the symptoms of hypoglycaemia, you need to raise your blood glucose urgently. You can do this by taking one of the following:
- 5-7 jelly beans
- 3 teaspoons of sugar or honey
- 1/2 can of ordinary (non-diet) soft drink
- 2-3 concentrated glucose tablets
- unless you are within 10 to 15 minutes of your next meal or snack, follow up with extra carbohydrates e.g. plain biscuits, fruit or milk - when over the initial symptoms. Taking this extra carbohydrate will prevent a second drop in your blood glucose level.
Make sure you, your friends, family and work colleagues can recognise the symptoms of hypoglycaemia and know how to treat them.
Your doctor will need to perform the following tests during treatment with Plaquenil:
Eye Tests
Your doctor will need to perform some eye tests every few months to check that your eyesight is not changing.
In extremely rare cases, Plaquenil has been associated with blindness. This can be avoided by having regular eye tests.
It is recommended you wear sunglasses when out in the sun.
Blood Tests
Your doctor will need to perform occasional blood tests to check for any blood reactions.
Your doctor may monitor your blood sugar levels if you have experienced hypoglycaemia while taking Plaquenil.
Driving/Operating Machinery
Be careful driving or operating machinery until you know how Plaquenil affects you. Plaquenil may cause problems with the eyesight of some people. Make sure you know how you react to Plaquenil before you drive a car, operate machinery, or do anything else that could be dangerous with blurred vision.
Side Effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while taking Plaquenil.
Plaquenil helps most people with rheumatoid arthritis, SLE, DLE, treatment of malaria and the control of malaria symptoms, but it may have unwanted side effects in a few people.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Ask your doctor or pharmacist to answer any questions.
Tell your doctor if you notice any of the following and they worry you:
Less serious side effects
Stomach problems such as:
- Nausea
- Vomiting
- Diarrhoea
- Abdominal cramps
Other problems such as:
- Loss of appetite
- Muscle weakness
- Dizziness
- Ringing in the ears
- Headache
- Nervousness
- Skin rash and itching
- Hair loss
If you already have psoriasis, you are more likely to experience skin reactions than other people when taking Plaquenil.
More serious side effects
Tell your doctor if you notice any of the following:
- Visual disturbances
- Any hearing loss
- Suicidal behaviour
- Frequent fevers, severe chills, bruising, sore throat or mouth ulcers (these may be signs of blood reactions)
- Changes in the way your heart beats
- More severe symptoms of hypoglycaemia, including:
- disorientation
- seizures, fits or convulsions
- loss of consciousness
These are serious side effects. You may need urgent medical attention.
Serious side effects are rare.
Tell your doctor if you notice anything else that is making you feel unwell. Some people may get other side effects while taking Plaquenil.
After taking Plaquenil
Storage
Keep your tablets in the bottle until it is time to take them. If you take the tablets out of the bottle they will not keep well.
Keep it in a cool dry place where the temperature stays below 25°C. Heat and dampness can destroy some medicines. Do not leave Plaquenil in the car on hot days.
Do not store Plaquenil or any other medicine in the bathroom or near a sink.
Keep Plaquenil where young children cannot reach it.
Children are particularly sensitive to the unwanted effects of Plaquenil.
A locked cupboard at least one and a half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking the tablets, ask your pharmacist what to do with any tablets that are left over.
Product Description
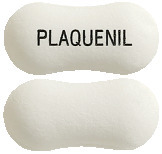
What Plaquenil looks like
Plaquenil comes as white to off-white peanut shaped tablets marked "PLAQUENIL" with black ink. A bottle contains 100 tablets.
Ingredients
Active Ingredient
Each Plaquenil tablet contains 200mg hydroxychloroquine sulfate.
Other ingredients
- Calcium Hydrogen Phosphate Dihydrate
- Starch-Maize
- Magnesium Stearate
- Water-Purified
- hypromellose
- Macrogol 400
- Titanium dioxide
- Polysorbate 80
- Carnauba Wax
- Black Ink
Australian Registration Number
AUST R 50055
Supplier
Plaquenil is supplied in Australia by:
sanofi-aventis australia pty ltd
12-24 Talavera Road
Macquarie Park NSW 2113
Tel: 1800 818 806
Plaquenil is supplied in New Zealand by:
sanofi-aventis new zealand limited
56 Cawley St
Ellerslie, Auckland, New Zealand
Phone: (09) 580 1810
This leaflet was prepared in April 2020.
plaquenil-ccdsv13-cmiv9-29apr20
Published by MIMS June 2020

 C18H26ClN3O, H2SO4.
C18H26ClN3O, H2SO4.