WHAT IS IN THIS LEAFLET
This leaflet answers some common questions about the QuitX Nicotine Transdermal Patch.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your pharmacist or doctor will be able to advise you about the risks and benefits of using QuitX.
If you have any concerns about using this medicine, ask your pharmacist or doctor.
Keep this leaflet with the medicine. You may need to read it again.
WHAT ARE QuitX PATCHES USED FOR
QuitX Patches can help you stop smoking. You probably know that smoking is a very difficult habit to break. There are two sides to quitting smoking. The first is the psychological dependence on cigarettes. You have probably smoked for many years and smoking has become an important part of your life.
The other side is the physical addiction to nicotine. Cigarettes contain nicotine, and your body has become dependent on nicotine. The QuitX patch delivers nicotine into your bloodstream through your skin. Using QuitX will help to gradually reduce the amount of nicotine your body craves each day.
Counselling is available from various groups such as Quitline. To get the best out of QuitX, we encourage you to enrol in one of these groups. QuitX is not intended for short periods, e.g. plane trips or other times when you cannot smoke. It is designed to help you quit smoking, not as a substitute for smoking.
QuitX works most effectively when you have a strong personal commitment to stop smoking. You cannot rely on QuitX alone to break the habit. Each nicotine patch helps relieve many nicotine withdrawal symptoms and cravings (such as early morning), which would otherwise have when you stop smoking
BEFORE YOU USE QuitX PATCHES
When you must not use it
Do not use QuitX patch if:
- you are under 12 years old
- you are a non-smoker
- you have a generalised skin disorder such as psoriasis or dermatitis
- you have an allergy to nicotine or to any component of the patch
- the expiry date printed on the carton or sachet has passed
- the packaging is torn or shows signs of tampering.
There are no health benefits to smoking. It is always better to give up smoking and using QuitX can help. In general, any possible side effects associated with nicotine replacement therapy (NRT) are far outweighed by the well-established dangers of continuing to smoke.
If you are in hospital because of a heart attack, severe heart rhythm disorders or a stroke, you should try to quit smoking without using NRT unless your doctor tells you to use it. Once you are discharged from the hospital, you may use NRT in consultation with your doctor.
If you have had allergic reactions that involve swelling of the lips, face and throat (angioedema) or itchy skin rash (urticaria), using NRT can sometimes trigger this type of reaction.
Before you start to use it
Talk to your doctor and ask for his/her advice before using QuitX Patch if:
- you have had a recent heart attack or stroke
- you have angina (chest pain) that is not well controlled or is getting worse
- you have severe arrhythmia (irregular heartbeat).
Talk to your doctor or pharmacist and ask for his/her advice before using QuitX Patch if:
- you have severe high blood pressure
- you have other heart or blood vessel disease
- you have diabetes
- you have kidney or liver problems
- you have hyperthyroidism (a disorder of the thyroid gland)
- you have phaeochromocytoma (a tumour of the adrenal gland)
- you are pregnant or breast-feeding
- you are aged 12 to 17 years.
If you require further advice, you should talk with your doctor or pharmacist.
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Your doctor and pharmacist will be able to advise you if stopping smoking may affect the way these medicines work.
Driving or operating machinery.
There is no evidence of any risk associated with driving or operating machinery if QuitX Patch is used according to the recommended dose but remember that smoking cessation can cause behavioural changes.
If you are pregnant.
Ideally you should not use this program while you are pregnant, and you should stop smoking without using nicotine replacement therapy. Nicotine in any form may cause harm to your unborn baby. However, if you are unable to quit without the use of nicotine replacement therapy, seek advice from your pharmacist or doctor before starting a program. The decision to use NRT should be made as early on in your pregnancy as possible and you should aim to use it for only 2-3 months. Products that are taken intermittently, such as gum, are preferable to nicotine patches. However, patches may be preferred if you have nausea or sickness.
If you are breast-feeding.
Nicotine is excreted in breast milk in quantities that may affect the child even in therapeutic doses. Like smoking, nicotine replacement therapy should be avoided during breast-feeding. However, if you are unable to quit without the use of nicotine replacement therapy, seek advice from your pharmacist or doctor before starting a program. Do not use QuitX Patch if you are breast-feeding. Intermittent NRT products such as nicotine chewing gums may be used instead, and should be used immediately after breast-feeding, to ensure that the baby gets the smallest amount of nicotine possible.
HOW TO GET STARTED on QuitX PATCHES
How to get started
It is probably best to nominate a specific day that you will stop smoking. It may be helpful to:
- Try to choose a day when you will not be where others are smoking.
- Choose a day with as little stress as possible.
- Choose a day not too far in the future.
Tell your family and friends that you have set this target "quit day".
This is the day you take complete control of your habit and become a non-smoker.
Prepare to stop smoking by trying to reduce the number of cigarettes you smoke each day. You should stop smoking completely while using QuitX.
How much to use
The QuitX course is a 12 week program and one patch is used each day for the duration of the course. Each patch is worn for one day (24 hours). As your course progresses, the aim is to use a lower strength (smaller size) patch as your body's desire for nicotine decreases. QuitX Patches come in 3 nicotine dosage strengths, QuitX Step 1 (21mg/24 hours), QuitX Step 2 (14mg/24 hours) and QuitX Step 3 (7mg/24 hours). The correct strength for you to start on depends on how many cigarettes you smoke each day.
Instructions are provided below for each of the two programs. Program A is for people who smoke 20 or more cigarettes each day and Program B is for people who smoke less than 20 cigarettes each day. Choose the correct program for you.
If you have a previous medical condition your doctor may choose to vary the dosage as appropriate.
Choose the program that is right for you.
Program A: If you smoke 20 or more cigarettes each day
Weeks 1 - 4:
- Stop cigarette smoking and use one QuitX Step 1 patch each day for 4 weeks.
- After 4 weeks you should no longer be smoking cigarettes and can move on to QuitX Step 2. However if you are still smoking, consult your doctor or pharmacist.
Weeks 5 - 8:
- Use one QuitX Step 2 patch each day for 4 weeks.
- After week 8, if you are still a non-smoker, you can move on to QuitX Step 3. But if you have smoked during weeks 5 - 8, consult your doctor or pharmacist.
Weeks 9 - 12:
- Use one QuitX Step 3 patch each day for another 4 weeks.
- After week 9 to 12 you should stop using the patches. You have become a non-smoker. Congratulations!
Program B: If you smoke less than 20 cigarettes each day
Weeks 1 - 4:
- Stop cigarette smoking and use one QuitX Step 2 patch each day for 4 weeks.
- After 4 weeks you should no longer be smoking cigarettes and can move on to QuitX Step 3. But if you are still smoking consult your doctor or pharmacist.
Weeks 5 - 12:
- Use one QuitX Step 3 patch each day for 8 weeks.
- If you have smoked during your course of treatment in weeks 5 - 8, please consult your doctor or pharmacist before proceeding further.
- Similarly, if you have smoked during your course of treatment in weeks 9 - 12, please consult your doctor or pharmacist.
- After week 9 to 12 you should stop using the patches. You have become a non-smoker. Congratulations!
| Program | Weeks 1-4 | Weeks 5-8 | Weeks 9-12 | End Program |
| A. If you smoked more than 20 cigarettes a day | Use QuitX Step 1 Patch 21 mg | Move to QuitX Step 2 Patch 14 mg | Move to QuitX Step 3 Patch 7 mg | Congratulations! You have successfully become a non-smoker |
| B. If you smoked less than 20 cigarettes a day | Use QuitX Step 2 Patch 14 mg | Move to QuitX Step 3 Patch 7 mg | Continue with QuitX Step 3 Patch 7 mg | Congratulations! You have successfully become a non-smoker |
Seek the advice of your doctor or pharmacist if you continue to have nicotine cravings after the 12 week program.
Combination therapy
If you have relapsed in the past or if you experience cravings while using a single form of nicotine replacement therapy (NRT), you can combine the use of QuitX patch with nicotine chewing gum 2 mg.
The combination is more effective than either product alone in people who have been unable to quit smoking using a single NRT method, increasing your chances of successfully quitting. When using QuitX Step 1 patch, chew one piece of nicotine chewing gum 2 mg if you get a craving. Use at least 4 pieces of gum and not more than 12 pieces in a day. Continue for 12 weeks.
After 12 weeks, you can wean yourself off therapy by either of the following methods:
- Stop use of QuitX Patch and gradually reduce the number of gums used until they are no longer needed.
- a. Use QuitX Step 2 patch for 3-4 weeks, while using the same number of pieces of nicotine chewing gum 2 mg in a day that you have routinely used.
b. Then use QuitX Step 3 for a further 3-4 weeks, while using the same number of pieces of nicotine chewing gum 2 mg in a day that you have routinely used.
c. When patch use is no longer needed, gradually reduce the number of gums you use until you no longer need them.
Adolescents 12 to 17 years old
QuitX patches should only be used in adolescents 12 to 17 years of age on the advice of a healthcare professional. Do not use for longer than 12 weeks. If you think you need to use for longer than 12 weeks, talk to your doctor or pharmacist.
Children
Do not use for children under 12 years.
HOW TO USE QuitX PATCHES
- Cut open the sachet along the dotted line. Keep the sachet for the future disposal of the patch.
- Remove the protective liner from the sticky side of the patch.
- Apply the QuitX patch to a clean, dry, non-hairy area of skin free from creams, lotions (including sunscreen products or insect repellents), ointments, oil or powder. Wash and clean the area thoroughly before application. After a warm bath or shower, wait until the skin is cool and dry before application of the patch (especially important in hot and humid weather, so as to maximise initial adhesion).
- Do not shave the skin as this could cause irritation.
- The skin should not be broken or inflamed in any way (this includes e.g. sunburn, rash, eczema).
- You may use your chest, back, upper arm or hip. Try to avoid areas where your skin folds when you sit or exercise.
- Place the sticky side of the patch onto the area of skin you have chosen and press firmly with the palm of your hand for at least 20 seconds. Then run your fingers around the edge pressing firmly. Do not try to check if the patch has stuck by lifting the edge. This may make it come loose.
- Once applied, do not remove and re-apply elsewhere as the patch will not re-adhere well.
- When replacing the QuitX patch after 24 hours, choose a different location for the new patch.
- Remove the used patch, fold it in half, sticky side inwards, replace in the original sachet and discard carefully, keeping it out of reach of children or pets.
- Do not flush down the toilet.
Additional Information
- You can swim, bath or shower with the patch on. However, wait at least one hour after you apply the patch before undertaking any sweaty or wet activity. This will help maximise patch adhesion.
- Do not use bath oils or shower gels with oily ingredients, either before or after application of the patch, as this could affect patch adhesion.
- Despite using all the precautionary measures noted in this leaflet, be aware that excessive sweating or oily skin can lead to poor patch adhesion. Very hot or humid conditions can also affect adhesion.
- If the patch should fall off, secure it back in place with a medicinal adhesive tape or apply a fresh patch and continue. Change the patch at the usual time the following day.
- It is best not to use soap on the patch or skin immediately surrounding the patch.
- Do not smoke while using the patch - remember there is nicotine in your system for several hours after removing the patch.
- If you forget to change the patch at the usual time, change it when you remember and then change the patch at the usual time the following day.
- It is recommended that you do not apply the patch to a previously used skin site for at least a week.
- You should wear no more than one QuitX patch at a time.
- QuitX Patches should not be used for periods longer than three months.
WHILE YOU ARE USING QuitX PATCHES
Things you must do
Use QuitX exactly as instructed. If you follow these recommendations you should get the full benefit of this QuitX program.
Stop smoking completely while using QuitX. You may have increased side effects if you continue to smoke while wearing the patch.
Tell your doctor or pharmacist if you continue to smoke while you are using QuitX.
Tell any other doctors, dentists, and pharmacists who treat you that you are using this medicine.
Things you must not do
Do not stop using QuitX Patches suddenly. You may get side effects similar to those you would get if you stopped smoking suddenly.
SIDE EFFECTS
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are using QuitX. All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- headache
- dizziness
- nausea (feeling sick)
- sleep disturbance.
These side effects are generally mild.
The patch can cause skin irritation. Using a different area of skin to apply the patch each day can reduce this.
However, if you have severe redness, swelling, itching, burning sensation or blisters at the patch site, or a rash (e.g. itchy red rash or hives) you should remove the patch and tell you doctor immediately or go to the Accident & Emergency at your nearest hospital. This could be an allergic reaction to QuitX.
Tell your doctor or pharmacist if you notice anything else that is making you feel unwell.
Keep QuitX Patches out of reach of children. Nicotine is a highly toxic substance and could be life threatening to children. Each QuitX patches sealed in a child-resistant sachet. Please take particular care to dispose of the used patches carefully.
IF YOU USE TOO MUCH (overdose)
If you smoke or use other products containing nicotine while wearing a QuitX patch, you may suffer an overdose of nicotine. However if used correctly, nicotine overdose is unlikely.
The signs and symptoms of nicotine overdosage include pallor, sweating, nausea, salivation, vomiting, stomach upset, diarrhoea, headache, dizziness, hearing and vision disturbances, tremor, confusion, weakness, fainting and breathing difficulties.
If overdosage is suspected, remove the patch immediately, wash the area liberally with water (do not use soap) and dry. Depending on the severity of the symptoms, seek advice from your pharmacist, doctor or Accident and Emergency at your nearest hospital.
Children: It is very important to keep both used and unused QuitX Patch out of the reach and sight of children, as doses of nicotine that are tolerated by adult smokers can cause severe poisoning in small children and can be fatal.
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital, in the event of overdose or suspected overdose.
AFTER USING QuitX PATCHES
Storage
Keep your patch in the sachet pack inside the carton until you are ready to use it. If you take the patch out of the sachet pack it may not keep well.
Store the patches below 25°C.
Do not store patches in the refrigerator, even in hot weather, as this could lead to a loss of adhesion.
Do not store it or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your pharmacist or doctor tells you to stop using the patch or the expiry date has passed, ask your pharmacist what to do with any patches that are left over.
PRODUCT DESCRIPTION
What it looks like
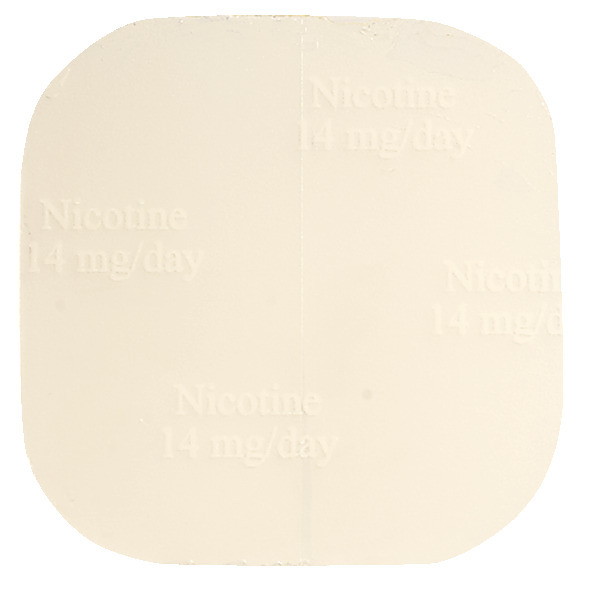
QuitX is available in 3 nicotine dosage strengths, each corresponding to a different patch size.
- QuitX nicotine 7 mg/24 hours, 9.8 cm2 square patch with round corners
- QuitX nicotine 14 mg/24 hours, 19.3 cm2 square patch with round corners
- QuitX nicotine 21 mg/24 hours, 29.0 cm2 square patch with round corners.
Available in cartons of 7, 14* and 28* sachets.
*Currently not marketed in Australia
Ingredients
Active Ingredients:
- QuitX nicotine 7 mg/24 hours - 7 mg nicotine per 24 hours
- QuitX nicotine 14 mg/24 hours - 14 mg nicotine per 24 hours
- QuitX nicotine 21 mg/24 hours - 21 mg nicotine per 24 hours
Inactive Ingredients:
- Polyethylene terephthalate
- DOW CORNING (R) BIO-PSA SA7-4207 silicon adhesive (ID: 108330)
- No-Tox liquid ink FGN-3274 NT15 White (ID: 108309)
- Loparex Si-600-1A adhesive (ID: 108412)
- Loparex Si-4400-1A adhesive (ID: 108351)
- Dura-Tak 87-2194 acrylic adhesive (ID: 108551).
Distributor
QuitX is distributed in Australia by:
Alphapharm Pty Limited
(ABN 93 002 359 739)
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
Phone: (02) 9298 3999
www.alphapharm.com.au
This leaflet was prepared in July 2013.
Australian Register Number(s)
21 mg/24 hours transdermal patch: AUST R 189708
14 mg/24 hours transdermal patch: AUST R 189707
7 mg/24 hours transdermal patch: AUST R 189706
Published by MIMS June 2015




 To sustain the concentration gradients for diffusion, more nicotine is contained in the nicotine patch than is actually delivered over 24 hours. Nicotine patch releases approximately 0.72 mg/cm2/24 hours of nicotine. Therefore, the average daily dose administered is determined largely by the size of the contact area of the system.
To sustain the concentration gradients for diffusion, more nicotine is contained in the nicotine patch than is actually delivered over 24 hours. Nicotine patch releases approximately 0.72 mg/cm2/24 hours of nicotine. Therefore, the average daily dose administered is determined largely by the size of the contact area of the system. The following unwanted experiences were also reported, irrespective of causal association with nicotine patches, at incidences of < 6%.
The following unwanted experiences were also reported, irrespective of causal association with nicotine patches, at incidences of < 6%.