What is in this leaflet
This leaflet answers some common questions about Renagel. It does not contain all the available information. It does not take the place of talking to your treating doctor or a trained health care professional.
All medicines have risks and benefits. Your treating doctor has weighed the risks of you taking Renagel against the benefits this medicine is expected to have for you.
If you have any concerns about taking this medicine, ask your treating doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What Renagel is used for
The name of your medicine is Renagel. It contains the active ingredient called sevelamer hydrochloride.
Renagel is used to treat hyperphosphataemia, a condition caused by too much dietary phosphorus being retained in your body due to a diseased kidney.
Ask your treating doctor if you have any questions about why this medicine has been prescribed for you.
How it works
Renagel belongs to a class of medicines that are called ion exchange resins.
Renagel helps to remove excess phosphorus that has built up in your body by binding the phosphorus that is in the food that you eat.
Before you use Renagel
When you must not take it
Do not take Renagel if you have an allergy to:
- any medicine containing sevelamer hydrochloride
- any of the ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include:
- shortness of breath, wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- skin rash, itching or hives
Do not take Renagel if you have:
- hypophosphatemia, a condition where you do not have enough phosphorus in your body
- a bowel obstruction
Do not take Renagel if the packaging is torn or shows signs of tampering.
Do not take Renagel after the expiry date (EXP) printed on the pack has passed.
If you take this medicine after the expiry date it may have no effect at all, or worse, an unexpected effect.
If you are not sure whether you should start taking this medicine, talk to your treating doctor.
Before you start to take it
You must tell your treating doctor if you have:
- allergies to any other medicines or substances such as foods, preservatives or dyes
- swallowing problems
- severe constipation
- problems with movement in your stomach and bowel
- active inflammation of the bowel
- undergone major surgery on your stomach or bowel
- you have or have had any other medical conditions, including a bowel obstruction or hypophosphatemia
- if you are pregnant or breast-feeding, think you may be pregnant on are planning to have a baby
If you have not told your treating doctor about any of the above, tell them before you take Renagel.
Taking other medicines
Tell your treating doctor if you are taking any other medicines, including medicines that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may be affected by Renagel or may affect how well it works. You may need different amounts of your medicine, or you may need to take different medicines. Your treating doctor will advise you.
The effects of medicines such as ciclosporin, mycophenolate mofetil and tacrolimus (medicines used to suppress the immune system) may be reduced by Renagel. Your doctor will advise you if you are taking these medicines.
Renagel should not be taken at the same time as ciprofloxacin (an antibiotic).
How to take Renagel
How much to take
Your treating doctor may initially prescribe 1 - 2 Renagel 800 mg tablets three times a day with your meals. Your doctor may adjust your dosage during treatment depending on your blood test results. The average daily dose of Renagel is 3 Renagel 800mg tablets per meal
Follow all directions given to you by your treating doctor and pharmacist carefully. These directions may differ from the information contained in this leaflet.
If you do not understand the instructions on the bottle, ask your treating doctor or pharmacist for help.
How to take it
Swallow Renagel tablets whole with a glass of water. Do not chew them. If you are having difficulty swallowing Renagel tablets, speak to your healthcare professional.
When the contents of Renagel come into contact with water they expand making them hard to swallow, therefore do NOT crush, chew or break the tablets into pieces before you take them.
How long to take it
Renagel helps lower your dietary phosphate. It does not cure your condition. Therefore, you must continue to take it as directed by your treating doctor if you expect to lower your phosphate level and keep it down. You may have to take phosphate-lowering medicine for the rest of your life. If you stop taking Renagel, your phosphate levels may rise again. It is important to keep taking your medicine even if you feel well.
Do not stop taking Renagel. Your treating doctor may need to change the dose of your other medicines if you stop Renagel, so you should only stop when your treating doctor tells you to.
If you forget to take it
If you miss a dose, do not take an extra dose to make up for the one you missed. Just go back to taking your tablets as you would normally.
It is important to try to take Renagel as prescribed by your treating doctor.
If you have trouble remembering to take your tablets, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your treating doctor or Poisons Information Centre [Australia telephone 13 11 26; in New Zealand telephone 0800 POISON or 0800 764 766], or go to accident and emergency at your nearest hospital, if you think that you or anyone else may have taken too much Renagel. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention. Keep telephone numbers of these places handy
While you are using Renagel
Things you must do
If you become pregnant while you are taking Renagel tell your treating doctor.
If you are about to be started on any new medicine tell your treating doctor and pharmacist that you are taking Renagel.
Be sure to keep all your doctor's appointments so your progress can be checked.
Your doctor will check your progress and monitor your phosphorus levels from time to time. This helps to ensure you are getting the right dose of Renagel.
Things you must not do
Do not give Renagel to anyone else, even if they have the same condition as you.
Do not use Renagel to treat any other complaints unless your treating doctor tells you to.
Do not stop taking your medicine or lower the dosage without checking with your treating doctor.
Side effects
Check with your treating doctor as soon as possible if you do not feel well or have any problems while taking Renagel, even if you do not think the problems are connected with the medicine or are not listed in this leaflet.
Like other medicines, Renagel can cause some side effects. If they occur, most are likely to be minor and temporary. However, some may be serious and need medical attention.
Ask your treating doctor or pharmacist any questions you may have.
Tell your treating doctor if you notice any of the following and they worry you:
- vomiting
- nausea
- constipation
- diarrhoea
- flatulence
- indigestion
- abdominal pain
- cold
- ongoing cough
- pain in limb or joint or back
- headache
- mechanical complication of implant
- loss of appetite
- high blood pressure.
Tell your doctor immediately if you notice any of the following symptoms:
- rash, itching or hives on the skin
- severe constipation
- vomiting blood or material that looks like coffee grounds, bleeding from the rectum, black sticky bowel motions (stools) or bloody diarrhoea
- fever or high temperature.
If any of the following happen, tell your doctor immediately or go to the Accident and Emergency at your nearest hospital:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
Other side effects not listed above may occur in some patients. Tell your treating doctor if you notice anything making you feel unwell when you are taking, or soon after you have finished taking, Renagel.
After using Renagel
Storage
Keep your tablets in the bottle until it is time to take them. If you take the tablets out of the bottle they may not keep well.
Keep Renagel in a cool dry place where the temperature stays below 25°C with the lid tightly closed. Do not put Renagel in the refrigerator. Do not store it or any other medicine in the bathroom or near a sink. Do not leave it in the car or on the window sills. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your treating doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
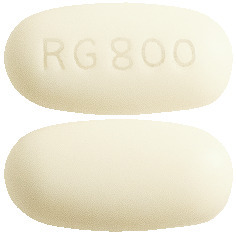
Renagel 800mg tablets are oval off-white film coated tablets imprinted with "Renagel 800" printed on one side and blank on the other side.
AUSTR 101553
Ingredients
Active ingredient: sevelamer hydrochloride
Other ingredients: colloidal anhydrous silica, stearic acid, OPADRY complete film coating system 06A29064 Clear, OPACODE WB monogramming ink NS-78-17715 BLACK.
Renagel does not contain gluten, lactose, sucrose, tartrazine or any other azo dyes.
Sponsor
In Australia this product is registered by:
sanofi-aventis australia pty ltd
12-24 Talavera Rd
Macquarie Park NSW 2113
Toll Free Number (medical information): 1800 818 806
E-mail: [email protected]
In New Zealand this product is distributed by:
sanofi-aventis new zealand limited
Level 8
56 Cawley Street
Ellerslie
Auckland
New Zealand
Toll Free Number (medical information): 0800 283 684
E-mail: [email protected]
This leaflet was prepared in February 2020
Further information is available via the App below.

Login code to access Renagel App: kidney
renagel-ccdsv6-cmiv15-25feb20
Published by MIMS May 2020

 When patients are converting from a calcium based phosphate binder, Renagel should be given in equivalent doses on a (mg to mg) weight basis compared to the patient's previous calcium based phosphate binder (see Table 2). Serum phosphorus levels should be closely monitored and the dose of Renagel adjusted accordingly with the goal of lowering serum phosphorus. Serum phosphorus should be tested every 2 to 3 weeks until a stable serum phosphorus level is reached, and on a regular basis thereafter.
When patients are converting from a calcium based phosphate binder, Renagel should be given in equivalent doses on a (mg to mg) weight basis compared to the patient's previous calcium based phosphate binder (see Table 2). Serum phosphorus levels should be closely monitored and the dose of Renagel adjusted accordingly with the goal of lowering serum phosphorus. Serum phosphorus should be tested every 2 to 3 weeks until a stable serum phosphorus level is reached, and on a regular basis thereafter.


 In a placebo controlled study with a treatment duration of two weeks, treatment emergent adverse events possibly or probably related to Renagel (N = 24) included dyspepsia (8.3%) and vomiting (4.2%). In a crossover study with treatment durations of eight weeks each, the treatment emergent events possibly or probably related to Renagel (N = 82) included dyspepsia (8.5%), diarrhoea (4.9%), nausea (4.9%), vomiting (4.9%), anorexia (3.7%), and gastrointestinal disorder (3.7%). In a long-term, open label extension trial the treatment emergent events possibly or probably related to Renagel (N = 192) included nausea (7.3%), abdominal pain (5.2%) and dyspepsia (4.7%).
In a placebo controlled study with a treatment duration of two weeks, treatment emergent adverse events possibly or probably related to Renagel (N = 24) included dyspepsia (8.3%) and vomiting (4.2%). In a crossover study with treatment durations of eight weeks each, the treatment emergent events possibly or probably related to Renagel (N = 82) included dyspepsia (8.5%), diarrhoea (4.9%), nausea (4.9%), vomiting (4.9%), anorexia (3.7%), and gastrointestinal disorder (3.7%). In a long-term, open label extension trial the treatment emergent events possibly or probably related to Renagel (N = 192) included nausea (7.3%), abdominal pain (5.2%) and dyspepsia (4.7%). Figure 1 illustrates that the proportion of patients achieving a given level of serum phosphorus lowering is comparable between the two treatment groups. For example, about half the patients in each group had a decrease of at least 0.65 mmol/L at endpoint. Successful control of serum phosphorus in chronic kidney disease patients is multifactorial including reduction of dietary phosphate intake, removal of phosphate with dialysis and inhibition of intestinal phosphate absorption with phosphate binders. As seen in Figure 1, some of the patients in GTC-36-301 did not respond to sevelamer treatment. Not all patients achieve phosphorus control with sevelamer alone, especially at the doses administered in this study (average actual daily dose 4.3 g/day). Later studies which employed higher doses of sevelamer (i.e. GTC-49-301 average actual daily dose 6.5 g/day) had a better rate of phosphorus response.
Figure 1 illustrates that the proportion of patients achieving a given level of serum phosphorus lowering is comparable between the two treatment groups. For example, about half the patients in each group had a decrease of at least 0.65 mmol/L at endpoint. Successful control of serum phosphorus in chronic kidney disease patients is multifactorial including reduction of dietary phosphate intake, removal of phosphate with dialysis and inhibition of intestinal phosphate absorption with phosphate binders. As seen in Figure 1, some of the patients in GTC-36-301 did not respond to sevelamer treatment. Not all patients achieve phosphorus control with sevelamer alone, especially at the doses administered in this study (average actual daily dose 4.3 g/day). Later studies which employed higher doses of sevelamer (i.e. GTC-49-301 average actual daily dose 6.5 g/day) had a better rate of phosphorus response. Average daily consumption at the end of treatment was 4.9 g sevelamer (range of 0.0 to 12.6 g) and 5.0 g of calcium acetate (range of 0.0 to 17.8 g). During calcium acetate treatment, 22% of patients developed serum calcium ≥ 2.75 mmol/L on at least one occasion versus 5% for sevelamer (p < 0.05). Thus the risk of developing hypercalcaemia is less with Renagel compared to calcium acetate.
Average daily consumption at the end of treatment was 4.9 g sevelamer (range of 0.0 to 12.6 g) and 5.0 g of calcium acetate (range of 0.0 to 17.8 g). During calcium acetate treatment, 22% of patients developed serum calcium ≥ 2.75 mmol/L on at least one occasion versus 5% for sevelamer (p < 0.05). Thus the risk of developing hypercalcaemia is less with Renagel compared to calcium acetate. Figure 2, a plot of the phosphorus change from baseline for the completers, illustrates the durability of response for patients who are able to remain on treatment.
Figure 2, a plot of the phosphorus change from baseline for the completers, illustrates the durability of response for patients who are able to remain on treatment. Average daily consumption at the end of the treatment was 6.5 g of sevelamer (range of 0.8 to 13 g) or approximately eight 800 mg tablets (range of 1 to 16 tablets), 4.6 g of calcium acetate (range of 0.7 to 9.5 g) and 3.9 g of calcium carbonate treatment, 34% of patients developed serum calcium corrected for albumin ≥ 2.75 mmol/L on at least one occasion versus 7% for Renagel (p < 0.05). Thus the risk of developing hypercalcaemia is less with Renagel compared to calcium salts.
Average daily consumption at the end of the treatment was 6.5 g of sevelamer (range of 0.8 to 13 g) or approximately eight 800 mg tablets (range of 1 to 16 tablets), 4.6 g of calcium acetate (range of 0.7 to 9.5 g) and 3.9 g of calcium carbonate treatment, 34% of patients developed serum calcium corrected for albumin ≥ 2.75 mmol/L on at least one occasion versus 7% for Renagel (p < 0.05). Thus the risk of developing hypercalcaemia is less with Renagel compared to calcium salts. Sevelamer, or poly(allylamine-co-N,N'-diallyl -1,3-diamino-2-hydroxypropane), a cross-linked polymer. The primary amine groups shown in the structure are derived directly from poly(allylamine hydrochloride). The crosslinking groups consist of two secondary amine groups derived from poly(allylamine hydrochloride) and one molecule of epichlorohydrin.
Sevelamer, or poly(allylamine-co-N,N'-diallyl -1,3-diamino-2-hydroxypropane), a cross-linked polymer. The primary amine groups shown in the structure are derived directly from poly(allylamine hydrochloride). The crosslinking groups consist of two secondary amine groups derived from poly(allylamine hydrochloride) and one molecule of epichlorohydrin.