What is in this leaflet
This leaflet answers some common questions about RESPRIM.
It does not contain all of the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have benefits and risks. Your doctor has weighed the risks of you taking RESPRIM against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, talk to your doctor or pharmacist.
Keep this leaflet with your medicine. You may need to read it again.
What RESPRIM is used for
RESPRIM contains the active ingredients sulfamethoxazole and trimethoprim, also known as co-trimoxazole.
RESPRIM is used to treat infections in different parts of the body caused by bacteria.
RESPRIM belongs to a group of medicines called antibiotics. There are many different types of medicines used to treat bacterial infections. Sulfamethoxazole in RESPRIM belongs to a group of medicines known as sulfonamides. Trimethoprim belongs to a group of medicines known as the benzylpyrimidines.
RESPRIM works by stopping the growth of the bacteria that is causing your infection.
RESPRIM will not work against infections caused by viruses, such as colds or flu.
RESPRIM has been prescribed for your current infection. Another infection later on may require a different medicine.
Your doctor may have prescribed RESPRIM for another reason.
Ask your doctor if you have any questions about why RESPRIM has been prescribed for you.
RESPRIM is available only with a doctor's prescription.
Before you take RESPRIM
When you must not take it
Do not take RESPRIM if:
- you have had an allergic reaction to trimethoprim, sulfamethoxazole or any other sulfonamide (sulfur) antibiotic, or any of the ingredients listed at the end of this leaflet
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue which may cause difficulty in swallowing or breathing
- rash, itching or hives, peeling of the skin. - you have severe liver or kidney disease, any blood disorder or megaloblastic anaemia
- the child you are treating is under 3 months of age
- you have streptococcal pharyngitis
- you are taking dofetilide, a medicine used to treat irregular heartbeats
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if:
- you are pregnant or intend to become pregnant.
If RESPRIM is taken during pregnancy, it may harm the baby.
Your doctor will discuss the risks and benefits of taking RESPRIM during pregnancy. - you are breastfeeding or wish to breastfeed.
RESPRIM passes into breast milk. Your doctor will discuss the risks and benefits of taking RESPRIM when breastfeeding. - you have any medical conditions, especially the following:
- you are allergic to:
- diuretics (fluid tablets)
- medicines used to treat diabetes
- medicines for an overactive thyroid.
You may have an increased chance of being allergic to RESPRIM if you are allergic to these medicines. - any type of blood disorder (including porphyria and glucose-6-phosphate dehydrogenase deficiency)
- liver or kidney disease
- a hereditary disorder called phenylketonuria
- epilepsy (fits or convulsions)
- asthma
- allergic disorders
- rheumatoid arthritis
- urinary obstruction
- folic acid deficiency
Tell your doctor if you are allergic to any other medicines, foods, dyes or preservatives.
If you have not told your doctor about any of the above, tell him/her before you start taking RESPRIM.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you buy without a prescription from a pharmacy, supermarket or health food shop.
Some medicines and RESPRIM may interfere with each other. These include:
- medicines used to treat diabetes, such as repaglinide, rosiglitazone, pioglitazone, glibenclamide, gliclazide, glipizide, chlorpropamide and tolbutamide
- diuretics (fluid tablets)
- phenytoin, a medicine used to treat epilepsy
- pyrimethamine, a medicine used to prevent malaria
- other medicines used to treat infections such as rifampicin, dapsone and polymyxin
- zidovudine, a medicine to treat HIV infection
- ciclosporin, a medicine used to prevent organ transplant rejections or to treat certain problems with the immune system
- warfarin, acenocoumarol, phenprocoumon, medicines used to thin the blood
- medicines used to treat some heart conditions such as digoxin and amiodarone
- amantadine, a medicine commonly used to treat the influenza virus and Parkinson's disease
- memantine, a medicine used to treat Parkinson's disease
- lamivudine, an antiretroviral medicine used to treat HIV/AIDS
- urinary acidifiers (for kidney conditions)
- oral contraceptives ('the pill')
- sulfinpyrazone, a medicine used to treat gout
- salicylates, medicines to treat conditions such as psoriasis or warts
- medicines used to treat cancer such as paclitaxel, mercaptopurine and methotrexate,
- clozapine, a medicine used to treat schizophrenia
- medicines used to treat overactive thyroid conditions
- medicines used to treat depression such as imipramine, clomipramine, amitriptyline, dosulepin (dothiepin), doxepin, nortriptyline and trimipramine
- immunosuppressant medicines such as azathioprine and methotrexate
- medicines used to treat high blood pressure as well as a variety of heart and kidney conditions such as captopril, enalapril, lisinopril, fosinopril, perindopril, quinapril, ramipril, trandolapril, valsartan, telmisartan, irbesartan, candesartan, eprosartan, losartan, dofetilide and olmesartan.
These medicines may be affected by RESPRIM or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines. Your doctor will advise you.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking RESPRIM.
If you have not told your doctor about any of the above, tell them before you start taking RESPRIM.
Use in very young children
RESPRIM should not be given to children under 12 years of age. Alternative dosage form of trimethoprim and sulfamethoxazole is available from other brands for children from 3 months to 12 years of age.
Use in People Over 65 Years
People over 65 years are more at risk of severe side effects when taking RESPRIM. The risk is greater if you have kidney or liver disease or are taking some types of other medicines, such as diuretics.
Use in People with HIV infection
People with HIV infection have been reported to get more side effects while being treated with RESPRIM than people without HIV.
How to take RESPRIM
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the pack, ask your doctor or pharmacist for help.
How much to take
Take RESPRIM exactly as your doctor has prescribed. Your doctor will tell you how much RESPRIM to take each day.
The dose and length of time you have to take RESPRIM will depend on the type of infection you have.
The usual dose for adults and children over 12 years is one RESPRIM FORTE tablet (or two RESPRIM tablets) twice a day.
How to take it
Swallow the tablets with a glass of water.
When to take it
Take RESPRIM after food. This will lessen the chance of a stomach upset.
How long to take it
Continue taking RESPRIM for as long as your doctor recommends.
Do not stop taking RESPRIM, even if you feel better after a few days, unless advised by your doctor. Your infection may not clear completely if you stop taking your medicine too soon.
If your symptoms do not improve within a few days, or if they become worse, let your doctor know.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take the missed dose as soon as you remember, and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for the dose you missed.
If you are not sure what to do, ask your doctor or pharmacist.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital, if you think you or anyone else may have taken too much RESPRIM. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
If you take too much RESPRIM, you may feel sick, vomit, feel dizzy, depressed or confused or have a headache. You may also feel drowsy or become unconscious.
Keep telephone numbers for these places handy.
If you are not sure what to do, contact your doctor or pharmacist.
While you are taking RESPRIM
Things you must do
Before starting any new medicine, tell your doctor or pharmacist that you are taking RESPRIM.
Tell all the doctors, dentists and pharmacists who are treating you that you are taking RESPRIM.
If you become pregnant while taking RESPRIM, tell your doctor.
Tell your doctor if, for any reason, you have not taken your medicine exactly as prescribed. Otherwise, your doctor may think that it was not effective and change your treatment unnecessarily.
Tell your doctor if you feel that RESPRIM is not helping your condition.
Drink plenty of fluids while you are taking RESPRIM. This will help to flush the medicine through your system.
If you are taking RESPRIM for a long time, visit your doctor regularly so your progress can be checked. Your doctor may ask you to have regular tests to check your kidneys, liver or blood.
If you have to have any blood tests, tell your doctor that you are taking RESPRIM. RESPRIM may affect the results of some tests.
Tell your doctor or pharmacist immediately if you get severe diarrhoea, even if it occurs several weeks after stopping RESPRIM. Do not take any diarrhoea medicine without checking with your doctor. Diarrhoea may mean that you have a serious condition affecting your bowel. You may need urgent medical care.
Things you must not do
Do not stop taking RESPRIM or change the dose without first checking with your doctor.
Do not let yourself run out of medicine over the weekend or on holidays.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not take RESPRIM to treat any other complaints unless your doctor tells you to.
Do not take any other medicines whether they require a prescription or not without first telling your doctor or consulting with a pharmacist.
Things to be careful of
Be careful driving or operating machinery until you know how RESPRIM affects you.
Sometimes use of this medicine allows other bacteria and fungi which are not sensitive to RESPRIM grow. If other infections such as thrush occur while you are taking RESPRIM tell your doctor.
Be careful drinking alcohol while taking RESPRIM. Combining RESPRIM and alcohol can make you feel sick, vomit, or have stomach cramps, headaches and flushing. Your doctor may suggest you avoid alcohol while being treated with RESPRIM.
Your skin may burn more easily while you are taking RESPRIM. If outdoors, wear protective clothing or use a SPF 30+ sunscreen.
Use in people with HIV infection:
People with HIV infection may not respond to RESPRIM in the same way as people without HIV, and have been reported to get more side effects.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking RESPRIM.
While you are taking RESPRIM
RESPRIM treats infections in most people, but it may have unwanted side effects in some people. All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Ask your doctor or pharmacist to answer any questions you may have.
If side effects do occur, they may be:
- nausea, with or without vomiting
- diarrhoea or other abdominal (gut) or stomach discomfort
These side effects are not usually serious or long lasting.
Tell your doctor if you notice these side effects and they worry you:
- oral thrush (white, furry sore tongue and mouth)
- vaginal thrush (sore and itchy vagina, vaginal discharge).
Your doctor will need to treat the thrush infection separately.
Tell your doctor immediately if you notice any of the following:
- jaundice (yellowing of the eyes or skin)
- severe or watery diarrhoea
- any type of skin rash, peeling of the skin, severe itching or hives
- fever, sore throat, lumps in the neck
- cough, shortness of breath
- severe persistent headache
- discolouration of urine
- renal stones
- swelling of the face and throat
These symptoms are usually rare but may be serious and need urgent medical attention.
Very rarely, people have died from complications due to certain severe skin, liver and blood reactions.
Elderly people, people with liver or kidney disease and people taking certain other medicines are more at risk of these severe reactions.
Other rare side effects include:
- other allergic reactions
- pins and needles in the hands & feet.
- Loss of appetite, fits, headaches, depression, imagined sensations or nervousness
- Increased or decreased urine production
- Unsteadiness or dizziness
- Sleeplessness, weakness, tiredness, increased sensitivity to light and stomach pains.
- uveitis (eye inflammation).
If you experience any of these effects contact your doctor as soon as possible.
This is not a complete list of all possible side effects. Others may occur in some people and there may be some side effects not yet known.
Tell your doctor if you notice anything else that is making you feel unwell, even if it is not on this list.
Ask your doctor or pharmacist if you don't understand anything in this list.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
After using RESPRIM
Storage
Keep your tablets in the pack until it is time to take them. If you take the tablets out of the pack they may not keep well.
Keep your tablets in a cool dry place where the temperature stays below 30°C.
Do not store RESPRIM or any other medicine in the bathroom or near a sink. Do not leave it in the car or on window sills. Heat and dampness can destroy some medicines.
Keep RESPRIM where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking RESPRIM, or your medicine has passed its expiry date, ask your pharmacist what to do with any that is left over.
Product description
What it looks like
RESPRIM tablets come in two strengths:
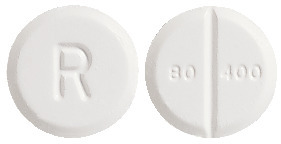
- RESPRIM - 11 mm flat bevelled edge white tablet marked, "80|400" on one side and "R" on the other side
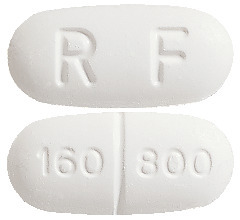
- RESPRIM FORTE - 19 mm x 9 mm oblong convex white tablet, marked "160|800" on one side and "RF" on the other side.
Each blister pack contains 10 tablets.
Ingredients
The active ingredients in RESPRIM tablets are trimethoprim and sulfamethoxazole:
- each RESPRIM tablet contains 80 mg of trimethoprim and 400 mg of sulfamethoxazole
- each RESPRIM FORTE tablet contains 160 mg of trimethoprim and 800 mg of sulfamethoxazole
RESPRIM and RESPRIM FORTE tablets contain the following inactive ingredients:
- povidone
- docusate sodium
- sodium starch glycollate
- magnesium stearate.
RESPRIM and RESPRIM FORTE tablets contain sulfites.
Supplier
RESPRIM and RESPRIM FORTE are supplied by:
Alphapharm Pty Ltd trading as Viatris
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.viatris.com.au
Phone: 1800 274 276
Australian registration numbers:
RESPRIM - AUST R 17681
RESPRIM FORTE - AUST R 17682
This leaflet was prepared in March 2024.
RESPRIM & RESPRIM FORTE_cmi\Mar24/00
Published by MIMS May 2024





 Molecular formula: C10H11N3O3S. Molecular weight: 253.3.
Molecular formula: C10H11N3O3S. Molecular weight: 253.3. Molecular formula: C14H18N4O3. Molecular weight: 290.3.
Molecular formula: C14H18N4O3. Molecular weight: 290.3.