What is in this leaflet
This leaflet answers some common questions about REXULTI.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking REXULTI against the benefits (s) he expects it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What REXULTI is used for
REXULTI is used to treat adults with schizophrenia.
Schizophrenia is a mental illness characterised by symptoms such as:
- hallucinations; hearing, seeing or sensing things that are not there
- suspiciousness, mistaken beliefs
- incoherent speech and behaviour, emotional flatness.
People with this condition may also feel depressed, guilty, anxious, or tense.
The cause of schizophrenia is not understood but medicines like REXULTI and other similar medicines help to control the symptoms of the disorder by their actions in the brain.
REXULTI belongs to a group of medicines called antipsychotic agents.
Although REXULTI cannot cure schizophrenia, it can help to keep the symptoms under control and reduce the risk of relapse as you continue treatment.
Ask your doctor if you have any questions about why this medicine has been prescribed for you.
REXULTI is available only with a doctor's prescription.
There is no evidence that REXULTI is addictive.
REXULTI should not be given to children and adolescents under 18 years of age as safety and efficacy have not been established in this age group.
Before you take REXULTI
When you must not take it
Do not take REXULTI if you have an allergy to:
- brexpiprazole, the active ingredient in REXULTI; or any of the other ingredients listed at the end of this leaflet under PRODUCT DESCRIPTION
- any other similar medicines (such as medicines of the same class).
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking REXULTI, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you are pregnant or plan to become pregnant. REXULTI is not recommended for use during pregnancy. If you need to take REXULTI during your pregnancy, your doctor will discuss the benefits and risks of taking it with you. The effect of REXULTI on your unborn baby is not known. Using REXULTI in the last three months of pregnancy may cause muscle movement problems, medicine withdrawal symptoms or both of these in a new-born.
Tell your doctor or pharmacist if you are breast-feeding or plan to breast-feed.
It is recommended that you do not breast-feed while taking REXULTI, as it is not known if REXULTI passes into breast milk and, therefore, there is a possibility that the breast-fed baby may be affected.
Tell your doctor if you have or have had any medical conditions, especially the following:
- dementia (loss of memory or other mental abilities) and you are elderly
- neuroleptic malignant syndrome - a reaction to some medicines with a sudden increase in body temperature, sweating, fast heartbeat, muscle stiffness and fluctuating blood pressure, which may lead to coma
- tardive dyskinesia - a reaction to some medicines with abnormal movements of the tongue, or other uncontrolled movements of the mouth, tongue, cheeks or jaw which may progress to the arms and legs
- high or low blood pressure
- rapid heartbeat and a drop in blood pressure when getting up
- problems with your heart or blood vessels, including stroke, "mini" stroke, or family history of heart or blood vessel problems
- epilepsy, seizures or fits
- suicidal thoughts or behaviour
- problems with your oesophagus (food pipe/gullet) such as difficulty in swallowing
- high blood sugar or diabetes mellitus or a family history of diabetes or high blood sugar
- high cholesterol, triglycerides, LDL - cholesterol or low levels of HDL-cholesterol
- blood clots, or you have any of the following risk factors for developing blood clots:
- family history of blood clots
- 65 years of age or older,
- smoking
- obese
- recent major surgery such as hip or knee replacement
- immobility due to air travel or other reason
- you take oral contraceptives - past experience with excessive gambling or other compulsive behaviours
- have or had low white blood cell count
- sleep apnoea (a sleep disorder where your breathing is interrupted during sleep).
Tell your doctor if you drink alcohol. You should avoid alcohol as it could magnify the side effects of this medicine.
If you have not told your doctor about any of the above, tell him/her before you start taking REXULTI.
Ask your doctor or pharmacist for advice before taking any medicine.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and REXULTI may interfere with each other. These include:
- medicines and herbal remedies used to treat depression and anxiety (e.g. Saint John's Wort, fluoxetine and paroxetine)
- anticonvulsants used to treat epilepsy or seizures
- medicines used to treat high blood pressure
- medicines used to treat heart rhythm disturbances
- medicines used to treat blood disorders (e.g. ticlopidine)
- medicines used to treat bacterial, fungal or viral infections (e.g. terbinafine and itraconazole).
These medicines may be affected by REXULTI or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
If you have not told your doctor or pharmacist about any of these things, tell them before you take REXULTI.
How to take REXULTI
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions, ask your doctor or pharmacist for help.
How much to take
Your doctor or pharmacist will tell you how much you will need to take each day. This will depend on your condition and whether you are taking any other medicines.
Follow the instructions they give you. The label put on the carton by the pharmacist will tell you how much you should take. If you take the wrong dose, REXULTI may not work as well as expected and your disease may not improve.
How to take it
REXULTI tablets should be swallowed whole and washed down with a glass of water.
REXULTI can be taken with or without food.
When to take it
Unless your doctor gives you other directions, you should take REXULTI only once a day.
Take your medicine at about the same time each day. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
How long to take it
Continue taking your medicine for as long as your doctor tells you.
This medicine helps to control your condition, but does not cure it. It is important to keep taking REXULTI even if you feel well.
If you forget to take it
Take your dose as soon as you remember, and continue to take it as you would normally.
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Do not take a double dose to make up for the dose that you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone Australia 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much REXULTI. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
While you are taking REXULTI
Things you must do
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking REXULTI.
Tell any other doctors, dentists, and pharmacists who treat you that you are taking REXULTI.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking REXULTI.
If you become pregnant while taking REXULTI, tell your doctor immediately.
Keep all of your doctor's appointments so that your progress can be checked.
Things you must not do
Do not take REXULTI to treat any other complaints unless your doctor tells you to.
Do not give REXULTI to anyone else, even if they have the same condition as you.
Do not stop taking REXULTI or lower the dosage without checking with your doctor. If you stop taking REXULTI suddenly, your condition may worsen. Your doctor may want you to gradually reduce the amount you are taking before stopping completely.
Things to be careful of
Be careful driving or operating machinery until you know how REXULTI affects you. This medicine may cause dizziness, light-headedness or tiredness in some people. If you have any of these symptoms, do not drive, operate machinery or do anything else that could be dangerous.
Avoid drinking alcohol while you are taking REXULTI.
You should avoid alcohol while taking REXULTI as it could magnify the side effects.
Make sure you keep cool in hot weather and keep warm in cool weather.
REXULTI may affect the way your body reacts to temperature changes. It may prevent sweating, even during heatwaves. You may feel dizzy or faint if you are too hot. To stay cool in hot weather, try to do the following:
- drink plenty of water
- wear light clothing
- spend time in air-conditioned environments (or keep windows open and use electric fans)
- take cool baths or showers and avoid hot baths and saunas
- try to restrict exercise or heavy work to cool parts of the day
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking REXULTI.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
If you are over 65 years of age you may have an increased chance of getting side effects.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you notice any of the following and they worry you:
- drowsiness
- diarrhoea
- indigestion
- weight increase
- shaking
- inability to stand or sit still; restless movement of the arms and legs such as tapping, marching in places, rocking, crossing and uncrossing legs
- feeling dizzy or fainting, especially when getting up from a lying or sitting position
- impulsive behaviours or urges (including gambling)
- sleep walking and related behaviours (including sleep related eating disorder).
Tell your doctor immediately, or go to Accident and Emergency at your nearest hospital, if you notice any of the side effects listed below. These side effects may occur rarely.
- serious allergic reaction (symptoms of an allergic reaction may include swelling of the face, lips, mouth or throat which may cause difficulty in swallowing or breathing, or rash, itching or hives)
- tardive dyskinesia - abnormal movements of the tongue, or other uncontrolled movements of the tongue, mouth, cheeks, or jaw which may progress to the arms and legs
- neuroleptic malignant syndrome - sudden increase in body temperature, sweating, fast heartbeat, muscle stiffness, high blood pressure and convulsions
- suicidal thoughts and actions or talk about death, suicide, self-harm or harm to others - pay close attention to any changes, especially sudden changes, in mood, behaviours, irritability, agitation, thoughts or feelings
- swelling, pain or redness in the leg, chest pain or difficulty in breathing
Elderly patients with dementia may suffer serious side effects such as stroke, pneumonia, or heart problems. These serious side effects can be life threatening.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some people.
After using REXULTI
Storage
Keep REXULTI in the original container.
If you take it out of its original container it may not keep well.
Keep REXULTI in a cool dry place where the temperature stays below 30°C.
Do not store REXULTI or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car.
Heat and dampness can destroy some medicines.
Keep it where children cannot reach it.
A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
REXULTI comes in six tablet strengths:
0.25 mg - Light brown, round, shallow convex, bevel-edged tablet, debossed with BRX and 0.25 on one side
0.5 mg - Light orange, round, shallow convex, bevel-edged tablet, debossed with BRX and 0.5 on one side
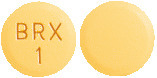
1 mg - Light yellow, round, shallow convex, bevel-edged tablet, debossed with BRX and 1 on one side
2 mg - Light green, round, shallow convex, bevel-edged tablet, debossed with BRX and 2 on one side
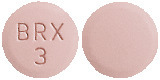
3 mg - Light purple, round, shallow convex, bevel-edged tablet, debossed with BRX and 3 on one side
4 mg - White, round, shallow convex, bevel-edged tablet, debossed with BRX and 4 on one side
REXULTI is available in blister packs of 30 tablets.
Ingredients
The active ingredient in REXULTI is brexpiprazole.
Inactive ingredients:
REXULTI tablets contain the following ingredients:
- lactose monohydrate
- maize starch
- microcrystalline cellulose
- hyprolose
- hyprolose - low-substituted
- magnesium stearate
- OPADRY complete film coating system 03A465005 BROWN
- OPADRY complete film coating system 03A430000 ORANGE
- OPADRY complete film coating system 03A420002 YELLOW
- OPADRY complete film coating system 03A410000 GREEN
- OPADRY complete film coating system 03A400000 PURPLE
- OPADRY complete film coating system 03A480004 WHITE
This medicine does not contain sucrose, gluten, tartrazine or any other azo dyes.
Sponsor
Lundbeck Australia Pty Ltd
Ground Floor,
1 Innovation Road
North Ryde NSW 2113
Ph: 02 8669 1000
REXULTI is co-marketed by Lundbeck Australia and Otsuka Australia Pharmaceutical:
Otsuka Australia Pharmaceutical Pty. Limited
Suite 2.03, Level 2, 9 Help Street,
Chatswood NSW 2067
Ph: 02 8021 9825
This leaflet was prepared on 24 January 2020.
Australian Registration Numbers:
Blister packs:
0.25 mg - AUST R 273225*
0.5 mg - AUST R 273222*
1 mg - AUST R 273220
2 mg - AUST R 273223
3 mg - AUST R 273221
4 mg - AUST R 273224
* Not currently marketed
REXULTI is a registered Trademark of H. Lundbeck A/S.
Published by MIMS April 2020






 Discontinuations due to TEAEs occurred at less than half the rate in the Rexulti group (5.2%) compared with the placebo group (11.5%) in the double blind maintenance phase of trial 3. No subjects discontinued due to weight gain or akathisia.
Discontinuations due to TEAEs occurred at less than half the rate in the Rexulti group (5.2%) compared with the placebo group (11.5%) in the double blind maintenance phase of trial 3. No subjects discontinued due to weight gain or akathisia.
 In trial 3, no clinically meaningful differences between treatment groups were observed for the incidences of metabolic parameters meeting potentially clinically relevant criteria.
In trial 3, no clinically meaningful differences between treatment groups were observed for the incidences of metabolic parameters meeting potentially clinically relevant criteria.
 In addition, Rexulti 2 mg/day and 4 mg/day demonstrated superiority to placebo in the key secondary endpoint, CGI-S score. Significant improvements in CGI-S score were seen as early as week 2 in trial 1 and week 3 in trial 2.
In addition, Rexulti 2 mg/day and 4 mg/day demonstrated superiority to placebo in the key secondary endpoint, CGI-S score. Significant improvements in CGI-S score were seen as early as week 2 in trial 1 and week 3 in trial 2. The long-term trial (trial 3) was a randomised, double-blind, placebo-controlled trial to assess the efficacy, safety, and tolerability of Rexulti 1-4 mg/day as maintenance treatment in adults with schizophrenia. The primary endpoint was the time to impending relapse. The key secondary endpoint was the percentage of subjects with impending relapse, and other secondary endpoints included the mean change in PANSS total score and CGI-S score.
The long-term trial (trial 3) was a randomised, double-blind, placebo-controlled trial to assess the efficacy, safety, and tolerability of Rexulti 1-4 mg/day as maintenance treatment in adults with schizophrenia. The primary endpoint was the time to impending relapse. The key secondary endpoint was the percentage of subjects with impending relapse, and other secondary endpoints included the mean change in PANSS total score and CGI-S score. The key secondary endpoint, the proportion of subjects who met the criteria for impending relapse, was statistically significantly lower with the Rexulti group compared with the placebo group in both the interim and final analyses (15.38% vs. 37.08%, p = 0.0016 and 13.54% vs. 38.46%, p < 0.0001, respectively).
The key secondary endpoint, the proportion of subjects who met the criteria for impending relapse, was statistically significantly lower with the Rexulti group compared with the placebo group in both the interim and final analyses (15.38% vs. 37.08%, p = 0.0016 and 13.54% vs. 38.46%, p < 0.0001, respectively). Examination of population subsets (age, race, and gender) on the primary endpoint did not reveal any differential responsiveness on the basis of these subgroupings.
Examination of population subsets (age, race, and gender) on the primary endpoint did not reveal any differential responsiveness on the basis of these subgroupings.

