1 Name of Medicine
Risedronate sodium hemipentahydrate.
2 Qualitative and Quantitative Composition
Each tablet contains 35, 75 or 150 mg risedronate sodium as the active ingredient.
Excipients with known effect.
Contains sugars as lactose.
For the full list of excipients see Section 6.1 List of Excipients.3 Pharmaceutical Form
Film coated tablets.
35 mg tablets.
Orange, round, biconvex coated tablets, engraved "APO" on one side, "RIS" over "35" on the other side.
75 mg tablets.
Dark pink, round, biconvex coated tablet, engraved "APO" on one side, "RIS" over "75" on the other side.
150 mg tablets.
Blue, round, biconvex coated tablet, engraved "APO" on one side, "RIS" over "150" on the other side.4.1 Therapeutic Indications
Treatment of osteoporosis.
Treatment of glucocorticoid-induced osteoporosis.
Preservation of bone mineral density in patients on long-term corticosteroid therapy.
4.2 Dose and Method of Administration
Risedronate tablets are intended for oral administration.
Dosage.
Risedronate must only be taken with plain water.
Plain water is the only drink that should be taken with risedronate tablets. Note that some mineral waters or water from regional areas may have a higher concentration of calcium and therefore should not be used.
Risedronate must be taken 30 minutes before the first food or drink other than water.
To facilitate delivery to the stomach, risedronate should be taken while the patient is in an upright position and the patients should avoid lying down for 30 minutes.
Patients should not chew or suck the tablet because of the potential for oropharyngeal irritation.
Osteoporosis.
The recommended dose is either:
5 mg daily;
35 mg once a week, taken on the same day each week;
75 mg taken for two consecutive days on the same dates each month; or
150 mg taken once a month. The tablet should be taken on the same date each month.
Patients who miss a 35 mg once a week dose should be instructed to take the missed dose on the day that it is remembered. Patients should then return to taking one tablet once a week on the day the tablet is normally taken. Two tablets should not be taken on the same day.
Patients who miss one or both 75 mg doses on two consecutive days per month should be instructed to take the missed dose or doses in the morning after the day it is remembered and the next morning, unless the time to the next month's scheduled doses are within 7 days. If the next month's scheduled doses are within 7 days, patients should wait until their next month's scheduled doses are due and then continue taking on two consecutive days each month as originally scheduled. Three tablets should not be taken in the same week.
Patients who miss a 150 mg once a month dose should be instructed to take the missed dose in the morning after the day it is remembered, unless the time to the next month's scheduled dose is within 7 days. If the next month's scheduled dose is within 7 days, patients should wait until their next month's scheduled dose is due and then continue taking as originally scheduled.
Renal impairment.
No dose adjustment is necessary in patients with mild to moderate renal insufficiency (creatinine clearance 30-60 mL/minute). Risedronate is not recommended in patients with severe renal impairment (creatinine clearance < 30 mL/minute) due to limited clinical data.
Hepatic impairment.
Dose adjustments are unlikely to be needed in patients with hepatic impairment.
Paediatric use.
Safety and efficacy of risedronate has not been established in patients under 18 years of age.
Use in the elderly.
No dosage adjustment is necessary.
Compatibility with other drugs.
Calcium, antacids, aluminium and some oral medications will interfere with the absorption of risedronate and therefore should be taken at a different time of the day.4.3 Contraindications
Known hypersensitivity to the drug or any of the ingredients in the tablets.
Hypocalcaemia (see Section 4.4 Special Warnings and Precautions for Use).
Inability to stand or sit upright for at least 30 minutes.
4.4 Special Warnings and Precautions for Use
General.
Food, certain medication containing polyvalent cations (such as calcium, magnesium, iron and aluminium) and beverages (except plain water) can interfere with the absorption of bisphosphonates and should not be taken at the same time as risedronate.
Bisphosphonates have been associated with oesophagitis, gastritis, oesophageal ulcerations and gastroduodenal ulcerations. Thus caution should be used:
In patients who have a history of oesophageal disorders which delay oesophageal transit or emptying e.g. stricture or achalasia.
In patients who are unable to stay in the upright position for at least 30 minutes after taking the tablet.
If risedronate is given to patients with active or recent oesophageal or upper gastrointestinal problems (including known Barrett's oesophagus).
For patients to gain maximum benefit from risedronate, doctors must stress the importance of taking risedronate as per the dosage instructions (see Section 4.2 Dose and Method of Administration). This is especially important in the case of patients with a history of oesophageal disorders.
Hypocalcaemia must be corrected before starting risedronate therapy.
Bone and mineral metabolism dysfunction (e.g. vitamin D deficiency and parathyroid abnormalities) should be effectively treated before starting risedronate therapy.
Patients should receive supplemental calcium and vitamin D if dietary intake is inadequate. This is especially important in patients with Paget's disease in whom bone turnover is significantly elevated.
Lactose.
This medicine contains lactose. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.
Gastrointestinal.
Risedronate, like other bisphosphonates, may cause local irritation of the upper GI mucosa. Since some bisphosphonates have been associated with oesophagitis and oesophageal ulcerations and gastroduodenal ulcerations, doctors should therefore be alert to any signs or symptoms signalling a possible oesophageal reaction, especially in patients with a history of upper GI disease or who are using NSAIDs or aspirin concomitantly. Doctors should be particularly careful to emphasise the importance of taking risedronate as per the dosage instructions to patients who have a history of oesophageal disorders.
There is very little experience with risedronate in patients with inflammatory bowel disease.
Osteonecrosis of the jaw.
Osteonecrosis of the jaw, generally associated with tooth extraction and/or local infection (including osteomyelitis) has been reported in patients with cancer receiving treatment regimens including primarily intravenously-administered bisphosphonates. Many of these patients were also receiving chemotherapy and corticosteroids. Osteonecrosis of the jaw has also been reported in patients with osteoporosis receiving oral bisphosphonates.
A dental examination with appropriate preventive dentistry should be considered prior to treatment with bisphosphonates in patients with concomitant risk factors (e.g. cancer, chemotherapy, radiotherapy, corticosteroids, poor oral hygiene).
While on treatment, these patients should avoid invasive dental procedures if possible. For patients who develop osteonecrosis of the jaw while on bisphosphonate therapy, dental surgery may exacerbate the condition. For patients requiring dental procedures, there are no data available to suggest whether discontinuation of bisphosphonate treatment reduces the risk of osteonecrosis of the jaw. Clinical judgment of the treating physician should guide the management plan of each patient based on individual benefit/ risk assessment.
Osteonecrosis of the external auditory canal.
Osteonecrosis of the external auditory canal has been reported with bisphosphonates, mainly in association with long-term therapy. Possible risk factors for osteonecrosis of the external auditory canal include steroid use and chemotherapy and/or local risk factors such as infection or trauma. The possibility of osteonecrosis of the external auditory canal should be considered in patients receiving bisphosphonates who present with ear symptoms including chronic ear infections.
Atypical stress fractures.
A small number of patients on long-term bisphosphonate therapy (usually longer than three years), mostly in connection with the use of alendronate have developed stress fractures of the proximal femoral shaft (also known as insufficiency or atypical fractures), some of which occurred in the absence of apparent trauma. Some of these patients experienced prodromal pain in the affected area, often associated with imaging features of stress fractures, weeks to months before a complete fracture occurred. Approximately one third of these fractures were bilateral; therefore the contralateral femur should be examined in patients who have sustained a femoral shaft stress fracture.
It is not known to what extent other agents of the aminobisphosphonate class, including risedronate sodium, may be associated with this adverse event. Prior treatment with alendronate should be a cause for added vigilance. Patients with suspected stress fractures should be evaluated, including evaluation for known causes and risk factors (e.g. vitamin D deficiency, malabsorption, glucocorticoid use, previous stress fracture, lower extremity arthritis or fracture, extreme or increased exercise, diabetes mellitus, chronic alcohol abuse), and receive appropriate orthopaedic care.
During bisphosphonate treatment patients should be advised to report any thigh, hip or groin pain and any patient presenting with such symptoms should be evaluated for an incomplete femur fracture.
Discontinuation of bisphosphonate therapy in patients with stress fracture is advisable pending evaluation of the patient, based on individual benefit/ risk assessment. Causality has not been excluded in regard to bisphosphonate use and stress fractures.
Osteomalacia.
The potential for risedronate to induce osteomalacia was investigated in the Schenk rat assay. This assay is based on histological examination of the epiphyses of the growing rats after drug treatment. Risedronate did not interfere with bone mineralisation, even at the highest dose tested (5 mg/kg/day, subcutaneously), which was > 3000 times the lowest anti-resorptive dose (1.5 microgram/kg/day). These data indicate that risedronate administered at therapeutic doses is unlikely to induce osteomalacia.
Use in renal impairment.
Risedronate is not recommended for use in patients with severe renal impairment (creatinine clearance < 30 mL/min).
Use in the elderly.
No dose adjustment is necessary.
Paediatric use.
Safety and efficacy of risedronate have not been established in patients under 18 years of age.
Effects on laboratory tests.
Bisphosphonates are known to interfere with the use of bone-imaging agents. However specific studies with risedronate have not been performed.
Small asymptomatic decreases in serum calcium and phosphorus levels have been observed in some patients.4.5 Interactions with Other Medicines and Other Forms of Interactions
No specific drug interactions studies have been performed. However risedronate is not systemically metabolised, does not induce or inhibit hepatic microsomal drug metabolising enzymes (e.g. cytochrome P450); it has low protein binding.
Concomitant intake of medications containing polyvalent cations (e.g. calcium, magnesium, iron, aluminium, antacids) will interfere with the absorption of risedronate and should be taken at a different time of the day.
Risedronate may be used concomitantly with hormone replacement therapy or the contraceptive pill.
During clinical trials, patients were exposed to a wide variety of commonly used concomitant medication while taking risedronate. No clinically relevant interactions were noted. The medications included NSAIDs, aspirin, H2-blockers, proton pump inhibitors, antacids, calcium channel blockers, beta-blockers, thiazides, glucocorticoids, anticoagulants, anticonvulsants and cardiac glycosides. There are no clinical data concerning the concomitant medication with two or more bisphosphonates and such concomitant medication is not recommended.
In the phase III post-menopausal trials with 5 mg daily dosing, 29% and 37% of patients used aspirin and NSAIDs, respectively. The incidence of upper GI adverse events in risedronate patients (aspirin/ NSAIDs taken ≥ 3 days/week) was similar to that in placebo treated patients. In the phase III once a week study, 57% and 40% of patients used aspirin and NSAIDs, respectively. In the phase III study comparing 75 mg taken on two consecutive days per month and 5 mg daily in post-menopausal women, acetyl salicylic acid/NSAID use was reported by 54.8% of patients. Similar percentages of patients experienced upper gastrointestinal adverse events regardless of NSAIDs and aspirin use.
4.6 Fertility, Pregnancy and Lactation
Effects on fertility.
A fertility study in male and female rats showed no adverse effects at oral doses up to 16 mg/kg/day, corresponding to systemic exposure (serum AUC0-24) about 30 times higher than that in humans dosed at 30 mg/day. At higher dose levels, systemic toxicity, testicular atrophy and reduced fertility were seen in male rats, but these effects are unlikely to have clinical relevance.
(Category B3)
Risedronate has not been studied in pregnant women. Risedronate should only be used during pregnancy if the potential benefit justifies the potential risk to mother and foetus. If administration during pregnancy is contemplated, serum calcium levels should be monitored and calcium supplementation provided in late gestation. Animal studies suggest that periparturient maternal hypocalcaemia and foetal ossification effects may occur.
Animal studies have shown that risedronate sodium crosses the placenta to a minimal extent in rats. The drug had no teratogenic activity in rats or rabbits at oral doses up to 80 and 10 mg/kg/day, respectively. However, suppression of foetal growth and retardation of ossification were observed at the highest dose level in rats. When administered to rats during late gestation, maternal deaths and parturition failure were observed at oral dose levels greater than 2 mg/kg/day. These effects were probably secondary to maternal hypocalcaemia. Systemic exposure (AUC0-24) at the no effect level in rats was about 6 times higher than that in patients with corticosteroid induced osteoporosis. Systemic exposure in rabbits was not measured.
Risedronate was detected in feeding pups exposed to lactating rats for a 24 hour period postdosing, indicating a small degree of lacteal transfer. It is not known whether risedronate is excreted in human milk. Due to the potential for serious adverse reactions in nursing infants from bisphosphonates, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
As with other bisphosphonates in preclinical models, foetuses from risedronate treated dams showed ossification changes in sternebrae and/or skull at doses as low as 3.2 mg/kg/day. This is equivalent to the human 30 mg dose and 6 times the human 5 mg dose based on surface area (mg/m2). Treatment with risedronate during mating and gestation with doses of 3.2 mg/kg/day has resulted in periparturient hypocalcaemia and mortality in rats allowed to deliver.4.7 Effects on Ability to Drive and Use Machines
The effects of this medicine on a person's ability to drive and use machines were not assessed as part of its registration. However, adverse effects of this medicine include dizziness, which could affect the ability to drive or use machines (see Section 4.8 Adverse Effects (Undesirable Effects)).
4.8 Adverse Effects (Undesirable Effects)
Risedronate-WGR sodium tablets are currently available only as 35 mg, 75 mg and 150 mg tablets.
Osteoporosis-risedronate 5 mg daily dosing.
The phase IIIA clinical trials were designed to include patients with a history of upper GI disorder. Patients were permitted concomitant use of NSAIDs and aspirin. In these patients the incidence of upper GI adverse reactions in the risedronate group was similar to that in the placebo control group.
Abdominal and musculoskeletal pain were commonly reported (1% to 10%). Glossitis, iritis and duodenitis were reported uncommonly (0.1% to 1%). There were rare reports (< 0.1%) of abnormal liver function tests.
Laboratory test findings.
Asymptomatic, small decreases in serum calcium and phosphorus levels have been observed in some patients.
Risedronate has been studied for up to 3 years in over 5000 women enrolled in phase III clinical trials for treatment or prevention of post-menopausal osteoporosis. Most adverse events reported in these trials were either mild or moderate in severity, and did not lead to discontinuation from the study. The incidence of serious adverse events in the placebo group was 24.9% and in the risedronate group was 26.3%. The percentage of patients who withdrew from the study due to adverse events was 14.4% and 13.5% for the placebo and risedronate groups, respectively.
Table 1 lists adverse events reported in ≥ 5% of risedronate treated patients and at an incidence higher than in the placebo group in phase 3 post-menopausal osteoporosis trials. Adverse events are shown without attribution of causality.

Endoscopic findings.
Risedronate clinical studies enrolled over 5000 post-menopausal women and included patients with pre-existing gastrointestinal disease and concomitant use of NSAIDs or aspirin. Investigators were encouraged to perform endoscopies in any patients with moderate to severe gastrointestinal complaints while maintaining the blind. These endoscopies were ultimately performed on equal numbers of patients between the treated and placebo groups [75 (11.9%) risedronate; 75 (14.5%) placebo]. Across treatment groups, the percentage of patients with normal oesophageal, gastric and duodenal mucosa on endoscopy was similar [20% placebo and 21% risedronate]. Positive findings on endoscopy were also generally comparable across treatment groups [58 (82.9%) placebo and 57 (81.4%) risedronate].
Gastrointestinal adverse events.
There was a higher number of reports of mild duodenitis [11 (15.7%)] in the risedronate group [7 (10%) placebo]; however, there were more duodenal ulcers [33 (47.1%)] in the placebo group [26 (37.1%) risedronate]. The number of patients who had positive findings and withdrew from the studies was similar across treatment groups [26 (37.1%) placebo and 27 (38.6%) risedronate] and there was no evidence of treatment related oesophageal, gastric or duodenal ulcers/ erosions.
Risedronate has been studied in phase III corticosteroid induced osteoporosis trials enrolling more than 500 patients. The adverse event profile in this population was similar to that seen in post-menopausal osteoporosis trials, except for musculoskeletal events, which were reported by > 10% of patients and occurred at a greater frequency in the risedronate 5 mg treatment group [75 (43.1%)] compared to the placebo group [57 (33.5%)]. The adverse experiences reported [165 placebo and 167 risedronate] have usually been mild or moderate and generally have not required discontinuation of treatment. The occurrence of adverse events does not appear to be related to patient age, gender, or race.
Osteoporosis-risedronate 35 mg once a week dosing.
In a one year, double blind, multi-centre study comparing risedronate 5 mg daily and risedronate once a week 35 mg in post-menopausal women with osteoporosis, the overall safety and tolerability profiles were similar. Table 2 lists the adverse events in > 5% of patients from this trial. Events are shown without attribution of causality.
 In a 2 year study in men with osteoporosis, the overall safety and tolerability were similar between the treatment and the placebo groups. Adverse experiences were consistent with those previously observed in women.
In a 2 year study in men with osteoporosis, the overall safety and tolerability were similar between the treatment and the placebo groups. Adverse experiences were consistent with those previously observed in women.
Osteoporosis-risedronate 75 mg on two consecutive days per month.
One year of treatment with risedronate 5 mg daily was compared to risedronate 75 mg two consecutive days per month in a double-blind, multi-centre study in post-menopausal women with osteoporosis. The overall safety and tolerability profiles of the two oral dosing regimens were similar. The incidence of serious adverse events was 4.7% in the risedronate 5 mg daily group and 7.5% in the risedronate 75 mg two consecutive days per month group. The percentage of patients who withdrew from treatment due to adverse events was 8.8% in the risedronate 5 mg daily group and 8.9% in the risedronate 75 mg two consecutive days per month group. Table 3 lists the adverse events in ≥ 2% of patients from this trial. Events are shown without attribution of causality.

Risedronate 150 mg once a month.
One year of treatment with risedronate 5 mg daily was compared to risedronate 150 mg once a month in a double-blind, multi-centre study in postmenopausal women with osteoporosis. The overall safety and tolerability profiles of the two oral dosing regimens were similar. The incidence of serious adverse events was 4.2% in the risedronate 5 mg daily group and 6.2% in the risedronate 150 mg once a month group. The percentage of patients who withdrew from treatment due to adverse events was 9.5% in the risedronate 5 mg daily group and 8.6% in the risedronate 150 mg once a month group. Table 4 lists the adverse events in ≥ 2% of patients from this trial. Events are shown without attribution of causality.

Acute phase reactions.
Acute phase reaction-like events, defined as adverse events of fever or influenza-like illness with onset within the first three days of treatment and duration of seven days or less, were reported by 9 (1.4%) patients on risedronate 150 mg once a month, and 1 (0.2%) patient on risedronate 5 mg daily.
Gastrointestinal adverse events.
The risedronate 150 mg once a month regimen resulted in a slightly higher incidence of discontinuation due to diarrhoea (0.8% versus 0.0%) compared to the risedronate 5 mg once daily regimen. All of these events occurred within a few days of the first dose. The incidence of vomiting that led to discontinuation was the same in both groups (0.3% versus 0.3%).
Ocular adverse events.
None of the patients treated with risedronate 150 mg once a month experienced ocular inflammation such as uveitis, scleritis or iritis; of patients treated with 5 mg daily, two patients reported iritis.
Laboratory test findings.
When risedronate 5 mg daily and risedronate 150 mg once a month were compared in post-menopausal women with osteoporosis, the mean percent changes from baseline at 12 months were 0.1% and 0.3% for serum calcium, -2.3% and -2.3% for phosphate and 8.3% and 4.8% for PTH, respectively. Compared to the risedronate 5 mg daily regimen, risedronate 150 mg once a month resulted in a slightly higher incidence of hypocalcaemia at the end of the first month of treatment (0.2%, 5 mg daily versus 2.2%, 150 mg). Thereafter, the incidence of hypocalcaemia with these regimens was similar at approximately 2%.
Other adverse events reported with use of risedronate in patients with Paget's disease.
Risedronate was studied in 392 patients with Paget's disease. The adverse events reported were usually mild or moderate and did not generally require discontinuation of treatment. There was no correlation between adverse events and the age or gender of the patient. In a double-blind, active controlled study, the adverse event profile was similar for risedronate and etidronate. 6.6% (4/61) of patients treated with risedronate 30 mg/day for 2 months discontinued treatment due to adverse events, compared with 8.2% (5/61) of patients treated with etidronate 400 mg/day for 6 months.
Adverse events reported in ≥ 5% of risedronate treated patients in the phase 3 study are shown in Table 5.
 Three patients that received risedronate 30 mg/day experienced acute iritis in one supportive study. All three patients recovered from their events; however, in one of these patients, the event recurred during risedronate treatment and again during treatment with pamidronate. All patients were effectively treated with topical steroids.
Three patients that received risedronate 30 mg/day experienced acute iritis in one supportive study. All three patients recovered from their events; however, in one of these patients, the event recurred during risedronate treatment and again during treatment with pamidronate. All patients were effectively treated with topical steroids.
In the phase 3 comparative study vs etidronate, patients with a history of upper GI disease or abnormalities and patients on NSAIDs or aspirin were also included. The proportion of risedronate treated patients [12 (19.7%)] with mild or moderate upper GI adverse events was similar to that in the etidronate treated group [12 (19.7%)]. No severe upper GI adverse events were observed in either group.
As expected the incidence of GI adverse events in patients who took concomitant NSAIDs or aspirin was higher than in non-users. However in these patients the incidence of GI adverse events was similar in the etidronate [10 (16.4%)] and risedronate [11 (18%)] treated patients.
Risedronate post-marketing data.
The following additional adverse reactions have been very rarely reported during postmarketing use:
Eye disorders.
Iritis, uveitis, orbital inflammation.
Musculoskeletal and connective tissues disorders.
Osteonecrosis of the jaw.
Skin and subcutaneous tissue disorders.
Hypersensitivity and skin reactions, including angioedema, generalised rash, and bulbous skin reactions, some severe.
Reporting suspected adverse effects.
Reporting suspected adverse reactions after registration of the medicinal product is important. It allows continued monitoring of the benefit-risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions at www.tga.gov.au/reporting-problems.4.9 Overdose
No specific information is available on the treatment of overdose with risedronate. Decreases in serum calcium following substantial overdose may be expected in some patients. Signs and symptoms of hypocalcaemia may also occur in some of these patients. Administration of milk or antacids (containing magnesium, calcium or aluminium) to chelate risedronate may be helpful. Standard procedures that are effective for treating hypocalcaemia, including the administration of calcium intravenously, would be expected to restore physiologic amounts of ionised calcium and to relieve signs and symptoms of hypocalcaemia.
For information on the management of overdose, contact the Poisons Information Centre on 131126 (Australia).
5 Pharmacological Properties
5.1 Pharmacodynamic Properties
Mechanism of action.
Risedronate is a potent pyridinyl bisphosphonate that binds to bone hydroxyapatite and inhibits osteoclast mediated bone resorption. Risedronate is a third generation bisphosphonate. In preclinical studies, risedronate demonstrated potent anti-osteoclast and anti-resorptive activity, increasing bone mass and biomechanical strength dose dependently. The activity of risedronate was confirmed by bone marker measurements during pharmacodynamic and clinical studies.
With risedronate sodium 5 mg daily, decreases in biochemical markers of bone turnover were observed within 1 month of treatment and reached a maximum decrease in 3-6 months, remaining stable during the course of therapy. This data demonstrates that risedronate causes a moderate reduction in bone resorption and bone turnover. The new steady state approximates the rate of bone turnover seen in pre-menopausal women. Decreases in biochemical markers of bone turnover were similar with risedronate sodium 35 mg once a week and 5 mg daily. In a study in men with osteoporosis, decreases in biochemical markers of bone turnover were observed at the earliest time point of 3 months and continued to be observed at 24 months.
Comparison of 5 mg once a day dose and 35 mg once a week dose.
Based on a lumbar spine bone mineral density (BMD), risedronate sodium 35 mg once a week (n = 485) was shown to be therapeutically equivalent to risedronate sodium 5 mg daily (n = 480) in a one year, double-blind multi-centre study of post-menopausal women with osteoporosis. The two treatment groups were also similar at one year with regard to BMD increases at the total proximal femur, femoral neck and trochanter.
Comparison of 5 mg daily dose and 75 mg on two consecutive days a month.
Based on effects on mean percent change in lumbar spine BMD, risedronate sodium 75 mg (n = 524) on two consecutive days a month was shown to be equivalent to risedronate sodium 5 mg (n = 527) daily in a one year, double blind multi-centre study of post-menopausal women with osteoporosis. Both groups had statistically significant mean percent increases from baseline to month 6, 12 and endpoint in lumbar spine BMD. The two treatment groups were also similar at one year with regard to BMD increases at the total proximal femur and trochanter. Swallowing the 75 mg tablet with hard water was shown to decrease bioavailability by about 60% compared with soft water.
Comparison of 5 mg once a day dose and 150 mg once a month dose.
Based on effects on mean percent change in lumbar spine BMD, risedronate sodium 150 mg once a month (n = 561) was shown to be equivalent to risedronate sodium 5 mg daily (n = 561) in a one year, double blind, multi-centre study of post-menopausal women with osteoporosis. Both groups had statistically significant mean percent increases in lumbar spine BMD from baseline to month 6, 12 and endpoint. The two treatment groups were also similar with regard to BMD increases at the total proximal femur and trochanter.
Clinical trials.
Treatment of osteoporosis.
The clinical program involved a wide range of early and late post-menopausal women with and without fracture, including those with a history of GI disease and those using aspirin, NSAIDs, proton pump inhibitors and H2-blockers.
The fracture efficacy of risedronate sodium 5 mg daily in the treatment of post-menopausal osteoporosis was demonstrated in two large, randomised, placebo-controlled, double-blind studies which enrolled a total of almost 4,000 women under similar protocols. The multinational study (RVE) was conducted primarily in Europe and Australia; a second study was conducted in North America (RVN). Patients were selected on the basis of radiographic evidence of previous vertebral fracture, and had established disease. The average number of prevalent vertebral fractures per patient at study entry was 4 in the multinational study and 2.5 in the North American study, with a broad range of baseline BMD levels. All patients in these studies received supplemental calcium 1000 mg/day. Patients with low vitamin D levels also received supplemental vitamin D 500 IU/day. The numbers of evaluable patients treated were:
RVN - 5 mg daily risedronate sodium n = 696; placebo n = 678.
RVE - 5 mg daily risedronate sodium n = 344; placebo n = 346.
RVN and RVE - 5 mg daily risedronate sodium n = 1040; placebo n = 1024.
Effect on vertebral fracture.
The pivotal studies of risedronate in the treatment of post-menopausal osteoporosis clearly demonstrate that risedronate sodium 5 mg daily reduces vertebral fracture incidence in patients with low bone mass and vertebral fractures, regardless of age, years since menopause or disease severity at baseline. Risedronate sodium 5 mg daily significantly reduced the risk of new vertebral fractures in each of the two large treatment studies. In the multinational study, treatment with risedronate sodium 5 mg daily for 3 years significantly reduced the risk of new vertebral fractures by 49% compared to treatment with placebo (p < 0.001) (Figure 1). A similar, significant reduction of 41% was seen in the North American study (p = 0.003). The effect of risedronate sodium 5 mg daily on vertebral fracture incidence was seen as early as the end of the first year of treatment in each study. In the multinational study, the incidence of new vertebral fractures after one year was reduced from 13.3 to 5.6%, an absolute risk reduction of 8% and a relative risk reduction of 61% (p < 0.001). In the North American study, the incidence of new vertebral fractures after one year was reduced from 6.4 to 2.4%, an absolute risk reduction of 4% and a relative risk reduction of 65% (p < 0.001). At both 1 and 3 years, the reduction in risk seen in the subgroup of patients who had 2 or more vertebral fractures at study entry was similar to that seen in the overall study population. Treatment with risedronate sodium 5 mg daily also significantly reduced the proportion of patients experiencing new and worsening vertebral fractures in each of the studies.

Effect on non-vertebral fractures.
In a prospectively planned analysis of pooled data from the multinational and North American studies, risedronate sodium 5 mg daily significantly reduced the cumulative incidence of patients experiencing osteoporosis-related non-vertebral fractures (wrist, humerus, clavicle, pelvis, hip and leg) over 3 years by 36% (p = 0.005). See Figure 2.
 The incidence of non-vertebral fractures in the pooled analysis (RVN and RVE) was lower in the risedronate sodium 5 mg daily group than in the placebo group for all fractures at these sites combined, as well as for the wrist, humerus, pelvis and leg separately. This difference was significant for all non-vertebral osteoporosis related fractures (p = 0.005), as well as for the humerus (p = 0.024) and pelvis (p = 0.044), while a trend was seen at the wrist (p = 0.075) (Table 6). These findings demonstrate a beneficial effect of risedronate in preventing non-vertebral, osteoporosis-related fractures.
The incidence of non-vertebral fractures in the pooled analysis (RVN and RVE) was lower in the risedronate sodium 5 mg daily group than in the placebo group for all fractures at these sites combined, as well as for the wrist, humerus, pelvis and leg separately. This difference was significant for all non-vertebral osteoporosis related fractures (p = 0.005), as well as for the humerus (p = 0.024) and pelvis (p = 0.044), while a trend was seen at the wrist (p = 0.075) (Table 6). These findings demonstrate a beneficial effect of risedronate in preventing non-vertebral, osteoporosis-related fractures.

Effect on height.
In the two 3 year osteoporosis treatment studies, standing height was measured yearly by stadiometer. As shown in Figure 3, treatment with risedronate sodium 5 mg daily was associated with a significant reduction of about 50% in the annual rate of height loss compared to treatment with placebo.

Effect on bone mineral density.
The results of four, large, randomised, placebo controlled trials in women with postmenopausal osteoporosis demonstrate that risedronate sodium 5 mg daily reverses the progression of disease, increasing BMD at the spine, hip and wrist compared to the effects seen with placebo. In the large multinational vertebral fracture treatment study previously described, risedronate sodium 5 mg daily produced increases in lumbar spine BMD which were progressive over at least 2 years of treatment and were statistically significant relative to baseline and to placebo at 6 months and at all later time points. The mean increase in BMD at the lumbar spine was 5.9%, compared to placebo at the end of 3 years. In the North American fracture trial, similarly progressive and significant increases were seen; the mean increase was 4.3%, compared to placebo. Risedronate sodium 5 mg daily also produced significant mean increases in BMD at the hip (femoral neck and trochanter) in each trial compared to losses in BMD in the placebo group. The increases compared to placebo were 3.1% at the femoral neck and 6.4% at the trochanter in the multinational study and 2.8% and 3.9%, respectively, in the North American study. Significant mean increases in the BMD of the midshaft radius, a skeletal site high in cortical bone, were also observed in each study in patients receiving risedronate treatment. These findings indicate that risedronate treatment produces positive effects at all measured skeletal sites of clinical importance for osteoporotic fractures.
Positive effects of risedronate treatment on BMD were also demonstrated in each of two large, randomised, placebo controlled trials in which almost 1200 postmenopausal women were recruited on the basis of low lumbar spine bone mass (more than 2 SD below the premenopausal mean) rather than a history of vertebral fracture. After 1½-2 years, risedronate produced significant mean increases in BMD of the lumbar spine compared to placebo (5% and 4.1% in the two studies), femoral neck (2.8% and 2.3%) and trochanter (3.3% and 3.3%) in these women with low bone mass.
Histology/ histomorphometry.
Histological evaluation of 278 bone biopsy samples from 204 post-menopausal women who received risedronate or placebo once daily for 2-3 years (including 74 pairs of biopsies, 43 from risedronate treated patients) showed a moderate decrease in bone turnover in risedronate treated women. Histological assessment showed no osteomalacia, impaired bone mineralisation or other adverse effects on bone in risedronate-treated women. These findings demonstrate that the bone formed during risedronate administration is of normal quality.
Bone markers.
In clinical studies, dose-dependent decreases in biochemical markers of bone turnover were observed with risedronate sodium 5 mg daily treatment. These effects were seen within 1 month of treatment and reached a plateau (with levels about 40% below baseline values) by the sixth month of treatment, which remained stable during continuous treatment for up to 3 years. These data demonstrate that risedronate sodium 5 mg daily causes a moderate reduction in bone resorption without over-suppression of bone formation. This new steady-state approximates the rate of bone turnover seen in pre-menopausal women.
Combined administration with hormone replacement therapy. The effects of combining risedronate sodium 5 mg daily with conjugated oestrogen treatment (0.625 mg daily) were compared to the effects of conjugated oestrogen alone in a 1 year, randomised, double blind study in more than 500 post-menopausal women (mean lumbar spine BMD 1.3 SD below the pre-menopausal mean). Risedronate sodium 5 mg daily in post-menopausal women taking oestrogen produced significant mean increases from baseline in BMD of the femoral neck (2.7%) and the midshaft radius (0.7%) at 12 months. These increases were greater than the increases observed in the oestrogen alone group and reached statistical significance in favour of the combined treatment at the femoral neck and midshaft radius.
Consistent with the changes in BMD, the reduction in bone turnover was significantly greater in the combined risedronate plus oestrogen group compared to the oestrogen alone group (40-47% versus 35-40%) and remained within the pre-menopausal range. Histological evaluation of 93 bone biopsy samples from 61 women on oestrogen therapy who received either placebo or risedronate once daily for one year (including 32 pairs of biopsies, 16 from risedronate treated patients) found decreases in bone turnover in the risedronate treated patients that were consistent with the changes in bone turnover markers. Bone histology demonstrated that the bone of patients treated with risedronate plus oestrogen was of normal lamellar structure and normal mineralisation.
Endoscopic findings.
Endoscopic findings from patients with moderate to severe GI complaints in both risedronate and control patients showed no evidence of treatment related gastric, duodenal or oesophageal ulcers. Duodenitis was rarely observed in the risedronate group. Four out of five patients with endoscopically-diagnosed oesophageal strictures had been taking risedronate sodium 5 mg daily for more than 6 months.
35 mg once a week dose. Risedronate sodium 35 mg once a week (n = 485) was shown to be therapeutically equivalent to risedronate sodium 5 mg daily (n = 480) in a 1 year, double-blind, multicentre study of post-menopausal women with osteoporosis. In the primary efficacy analysis of completers, the mean increases from baseline in lumbar spine BMD at one year were 4.0% (3.7-4.3; 95% CI) in the 5 mg daily group (n = 391) and 3.9% (3.6-4.3; 95% CI) in the 35 mg once a week group (n = 387) and the mean difference between 5 mg daily and 35 mg once a week was 0.1% (-0.42-0.55; 95% CI) (see Table 7). While once a week doses of risedronate resulted in slightly smaller increases in lumbar spine BMD compared to daily doses after 6 months, the two regimens were equivalent after 12 months. The clinical relevance of these 6 month BMD differences is unknown. The results of the intent to treat analysis with the last observation carried forward were consistent with the primary efficacy analysis of completers. The two treatment groups were also similar with regard to BMD increases at other skeletal sites. This study is of 2 years duration, the results of which will be included as soon as they are available.
 Very few patients in any treatment group had new fractured vertebrae at month 12 (5 mg daily: 1.5%; 35 mg once a week: 1.3%). No patient had more than one new fractured vertebra. There were no statistically significant differences in the percentage of patients with new vertebral fractures among the two treatment groups.
Very few patients in any treatment group had new fractured vertebrae at month 12 (5 mg daily: 1.5%; 35 mg once a week: 1.3%). No patient had more than one new fractured vertebra. There were no statistically significant differences in the percentage of patients with new vertebral fractures among the two treatment groups.
75 mg on two consecutive calendar days a month dose. Clinical equivalence has been demonstrated against a 5 mg daily dose of risedronate sodium. In a double-blind, multi-centre study of postmenopausal women with osteoporosis, 1 year of treatment with risedronate 75 mg two consecutive days/month (n = 616) was shown to be non-inferior to risedronate 5 mg daily (n = 613). In the primary efficacy analysis of completers, the mean increases from baseline in lumbar spine BMD at 1 year were 3.6% (3.3, 3.9; 95% CI) in the 5 mg daily group (n = 527) and 3.4% (3.1, 3.7; 95% CI) in the 75 mg two days/month group (n = 524) with a mean difference between groups being 0.2% (-0.2, 0.6; 95% CI). The results of the intent to treat analysis with the last observation carried forward were consistent with the primary efficacy analysis of completers. The two treatment groups were also similar with regard to BMD increases at other skeletal sites. The numbers of patients with new vertebral fractures at month 12 and endpoint were similar in the 75 mg two days month group and the 5 mg daily group (at endpoint, 75 mg two day month 1.09%; 5 mg daily 1.46%).
150 mg once a month dose. In a double-blind, active controlled, multi-centre study of post-menopausal women with osteoporosis, one year of treatment with risedronate sodium 150 mg once a month (n = 650) was shown to be non-inferior to risedronate sodium 5 mg daily (n = 642). In the primary efficacy analysis of completers, the mean increases from baseline in lumbar spine BMD at 1 year were 3.4% (3.0-3.8; 95% CI) in the 5 mg daily group (n = 561) and 3.5% (3.1-3.9; 95% CI) in the 150 mg once a month group (n = 561), with a mean difference between groups being -0.1% (-0.5-0.3; 95% CI). The two treatment groups were also similar with regard to BMD increases at other skeletal sites. The numbers of patients with new vertebral fractures at month 12 and at endpoint were similar in the 150 mg once a month group and the 5 mg daily group (at endpoint: 150 mg 1.4%; 5 mg 1.4%).
Treatment of osteoporosis in men.
Risedronate sodium 35 mg once a week demonstrated efficacy in men with osteoporosis (age range 36-84 years) in a 2 year, double blind, placebo controlled study in 284 patients (risedronate sodium 35 mg n = 191). All patients received supplemental calcium and vitamin D. The primary efficacy endpoint was assessed by the percentage change from baseline in lumbar spine BMD at endpoint (month 24 or last post-baseline observation). Secondary efficacy measures included lumbar spine and proximal femur BMD at 6, 12 and 24 months; BMD responders (defined as patients who had a positive lumbar spine BMD change at month 24); bone turnover markers at 6, 12 and 24 months; body height; incidence of new vertebral fractures and incidence of clinical fractures. Increases in BMD were observed as early as 6 months following initiation of risedronate sodium treatment. The primary analysis showed a statistically significant difference between risedronate and placebo in least squares mean percent change from baseline to endpoint (p < 0.0001). The estimated difference at endpoint between risedronate and placebo in the ITT population was 4.53% (95% CI: 3.46-5.60%). Risedronate sodium 35 mg once a week produced mean increases in BMD at the lumbar spine, femoral neck, trochanter and total hip compared to placebo after two years of treatment. The bone effect (BMD increase and BTM decrease) of risedronate is similar in males and females.
Corticosteroid-induced osteoporosis.
Bone mineral density.
Two 1 year, double-blind, placebo-controlled trials demonstrated that risedronate sodium 5 mg daily was effective in maintaining or increasing BMD in men and women initiating or continuing corticosteroid therapy.
The first study enrolled 228 patients, each of whom had initiated corticosteroid therapy (≥ 7.5 mg/day of prednisone or equivalent) within the previous 3 months for rheumatic, skin and pulmonary diseases. The mean lumbar spine BMD was normal at baseline. All patients in this study received supplemental calcium 500 mg/day. After one year of treatment, the placebo group lost BMD at the lumbar spine, femoral neck and trochanter, as shown in Figure 4. Risedronate sodium 5 mg daily prevented this bone loss with a statistically significant difference from placebo of 3.8% at the lumbar spine, 4.1% at the femoral neck and 4.6% at the trochanter. The results at these three sites were also statistically significant when the subgroups of men or post-menopausal women were analysed separately. Risedronate prevented bone loss regardless of underlying disease, age, race, gender, corticosteroid dose or baseline BMD.
 The effect of risedronate discontinuation on bone mineral density was studied in a double-blind, placebo-controlled study in post-menopausal women with glucocorticoid dependent rheumatoid arthritis. Women were treated for two years with risedronate 2.5 mg daily, cyclic risedronate (averaged 2.5 mg risedronate per day over the 96 week active period) or placebo and then followed without treatment for one more year. Patients continued glucocorticoid treatment during the third year of the study. Risedronate discontinuation resulted in bone loss at all skeletal sites (proximal femur and lumbar spine) during the third year. The rate of bone loss, however, was similar to the placebo group indicating that bone loss was not accelerated after risedronate was discontinued. The study supports the use of continuous treatment with risedronate to prevent bone loss.
The effect of risedronate discontinuation on bone mineral density was studied in a double-blind, placebo-controlled study in post-menopausal women with glucocorticoid dependent rheumatoid arthritis. Women were treated for two years with risedronate 2.5 mg daily, cyclic risedronate (averaged 2.5 mg risedronate per day over the 96 week active period) or placebo and then followed without treatment for one more year. Patients continued glucocorticoid treatment during the third year of the study. Risedronate discontinuation resulted in bone loss at all skeletal sites (proximal femur and lumbar spine) during the third year. The rate of bone loss, however, was similar to the placebo group indicating that bone loss was not accelerated after risedronate was discontinued. The study supports the use of continuous treatment with risedronate to prevent bone loss.
A second study of similar design enrolled 290 patients with continuing, long-term use (≥ 6 months) of corticosteroids for rheumatic, skin and pulmonary diseases. The baseline mean lumbar spine BMD was low (1.64 SD below the young healthy population mean), with 28% of the patients more than 2.5 SD below the mean. All patients in this study received supplemental calcium 1000 mg/day. Patients also received supplemental vitamin D 400 IU/day. After one year of treatment, the BMD of the placebo group remained near baseline levels at the lumbar spine, femoral neck and trochanter. Risedronate sodium 5 mg daily improved bone mass with a statistically significant mean increase compared to placebo of 2.7% at the lumbar spine and 1.9% at the femoral neck, as shown in Figure 5. At the trochanter, a statistically significant increase from baseline was demonstrated (2.4%). Risedronate was effective regardless of age, race, gender, underlying disease, corticosteroid dose or baseline BMD.

Vertebral fractures.
Vertebral fractures were monitored for safety in the two placebo controlled studies. The incidence of vertebral fractures in each study was 15-17% in the placebo patients. The risk of vertebral fractures was reduced approximately 70% in the patients treated with risedronate sodium 5 mg daily compared to patients treated with placebo. This decrease reached statistical significance when the studies were pooled, but not when analysed individually.
Bone marker data.
Risedronate sodium 5 mg daily produced significant reductions in biochemical markers of bone turnover relative to placebo. Deoxypyridinoline/ creatinine and bone specific alkaline phosphatase were significantly reduced by approximately 20% relative to placebo after 1 and 3 months of treatment, respectively and remained reduced (maximum 35% and 26%, respectively) for the duration of the treatment period.
Histology/ histomorphometry.
Histological evaluation of 70 bone biopsy samples from 48 women on corticosteroid therapy who received either placebo or risedronate once daily for one year (including 22 pairs of biopsies, 16 from risedronate treated patients) showed that bone formed during treatment with risedronate was of normal lamellar structure and normal mineralisation, with no bone or marrow abnormalities observed. Histomorphometric evaluation indicated that risedronate reduces bone resorption and produces a mild to moderate decrease in the rate of bone turnover. The rate of bone formation was preserved or increased and there was no evidence of impaired mineralisation. The structure of the cortical bone (cortical thickness and porosity) was maintained in the risedronate treated patients; cortical porosity increased, however, in the placebo group. These findings indicate that bone formed during risedronate treatment is of normal quality.
5.2 Pharmacokinetic Properties
Absorption.
Risedronate is relatively rapidly absorbed (tmax ~ 1 hour) throughout the upper gastrointestinal (GI) tract. Absorption is independent of dose over the range studied (single dose study, 2.5 to 30 mg; multiple dose studies, 2.5 to 5 mg daily and up to 150 mg once a month). In a 13 week pharmacokinetic study with 5 mg daily and 35 mg or 50 mg once a week dosing (n ~ 19/ group), a comparison of the average serum concentration (Cavg) for 35 mg once a week and 5 mg/day was not statistically significantly different. Steady-state conditions in the serum are observed within 57 days of daily dosing.
Mean oral bioavailability of the tablet is 0.63% and is decreased when risedronate is administered with food. Bioavailability was similar in men and women.
Although administration of risedronate either 30 minutes prior to breakfast or 2 hours after dinner reduces absorption of risedronate by 55% compared to administration in the fasting state (i.e. no food or beverages for 10 hours prior to or 4 hours after, dosing) and administration one hour prior to breakfast reduces absorption by 30%, risedronate has been shown to be effective in clinical trials when administered 30 minutes (or longer) before the first meal or beverage of the day (e.g. breakfast) and also when administered 2 hours (or longer) prior to and following food or beverages at other times of the day.
Distribution.
The mean steady-state volume of distribution is 6.3 L/kg in humans. Human plasma protein binding of risedronate is about 24%. Preclinical studies in rats and dogs dosed intravenously with single doses of [14C]-risedronate indicate that 40-45% of the dose was distributed in the bone after 72 hours. At the same time, risedronate levels in soft tissues of rats and dogs were at least 40 times and 16 times lower than those in bone, respectively. The remainder of the dose was mainly excreted in the urine. This is likely to be considerably lower in humans who excrete 65% of an intravenously administered dose in the urine in 24 hours. After multiple oral dosing in rats, accumulation of risedronate was observed in bone but not in soft tissues.
Metabolism.
There is no evidence of systemic metabolism of risedronate.
Excretion.
Approximately half the absorbed dose is excreted in the urine within 24 hours, whilst 85% of an intravenous dose is recovered in the urine over 28 days. Mean renal clearance is 105 mL/min and mean total clearance is 122 mL/min. The difference primarily reflects non-renal clearance or clearance due to adsorption to bone. The renal clearance is not concentration dependent and there is a linear relationship between renal clearance and creatinine clearance. In the same pharmacokinetic study mentioned in the "Absorption" section, the percent of dose excreted in urine was measured. The point estimate for the 35 mg once a week versus 5 mg daily doses was 66.8% (95% CI, 48.0-95.8). Although this was statistically significantly different, the clinical relevance is unknown.
Unabsorbed risedronate is eliminated unchanged in the faeces. Following absorption, the serum concentration time profile is multi-phasic with an initial half-life of about 1½ hours and a terminal exponential half-life of 480 hours. Although the elimination rate from human bone is unknown, the 480 hour half-life is hypothesised to represent the dissociation of risedronate from the surface of the bone.
Special groups.
Paediatric.
Safety and efficacy of risedronate have not been established in patients under 18 years of age.
Gender.
Bioavailability and pharmacokinetics following oral administration are similar in men and women.
Use in the elderly.
Risedronate pharmacokinetics are similar in older subjects (age 45 to 76 years) with normal renal function (creatinine clearance 80 to 120 mL/min) to that observed in young subjects (age 18 to 45 years). No dosage adjustment is necessary (see Section 4.2 Dose and Method of Administration).
Ethnicity.
Pharmacokinetic differences due to ethnicity have not been studied.
Renal insufficiency.
Risedronate is excreted intact primarily via the kidney. There is limited clinical data in patients with severe renal impairment (creatinine clearance < 30 mL/min) and therefore risedronate is not recommended for this patient group. No dosage adjustment is necessary in patients with a creatinine clearance ≥ 30 mL/min.
Hepatic insufficiency.
No studies have been performed to assess the safety or efficacy of risedronate in patients with hepatic impairment. Risedronate is not metabolised in rat, dog, and human liver preparations. Insignificant amounts (< 0.1% of intravenous dose) of risedronate are excreted in the bile in rats. Therefore, dosage adjustment is unlikely to be needed in patients with hepatic impairment.
5.3 Preclinical Safety Data
Genotoxicity.
Risedronate did not cause gene mutations in bacterial or mammalian cells in vitro, nor did it cause DNA damage in rat hepatocytes in vitro. In clastogenicity assays, risedronate was positive in an in vitro assay using Chinese hamster ovary cells at cytotoxic concentrations (7-18% cell survival), but there was no evidence of chromosomal damage when the assay was repeated at concentrations leading to 48-74% cell survival. Risedronate was negative at oral doses up to 1336 mg/kg in an in vivo assay (chromosomal aberrations in rat bone marrow).
Carcinogenicity.
No evidence of carcinogenicity was observed in either rats (treated for 104 weeks with up to 24 mg/kg/day) or mice (treated for 80 weeks with up to 32 mg/kg/day). Systemic exposure (serum AUC0-24) at the high dose in rats was 160 times greater than that in humans dosed at 30 mg/day. Systemic exposure was not assessed in mice, but the highest dose in the carcinogenicity study was at least 30 times higher than the dose required for pharmacological effects on bone. Thus, risedronate appears to have no carcinogenic potential at therapeutic dose levels.6 Pharmaceutical Particulars
6.1 List of Excipients
Lactose monohydrate, crospovidone, magnesium stearate, hypromellose, macrogol 8000, hyprolose, colloidal anhydrous silica, titanium dioxide, iron oxide red (35 mg and 75 mg tablets only), iron oxide yellow (35 mg tablets only), indigo carmine aluminium lake (150 mg tablets only), monobasic sodium phosphate dihydrate (150 mg tablets only), dibasic sodium phosphate (150 mg tablets only).
6.2 Incompatibilities
Incompatibilities were either not assessed or not identified as part of the registration of this medicine.
6.3 Shelf Life
In Australia, information on the shelf life can be found on the public summary of the Australian Register of Therapeutic Goods (ARTG). The expiry date can be found on the packaging.
6.4 Special Precautions for Storage
Store below 25°C.
6.5 Nature and Contents of Container
Risedronate-WGR tablets.
35 mg tablets.
Blister pack (clear PVC/PVDC/Aluminium silver foil) of 1, 4, 8, 12, 16 tablets.
75 mg tablets.
Blister pack (clear PVC/PVDC/Aluminium silver foil) of 2, 4, 6, 8 tablets.
150 mg tablets.
Blister pack (clear PVC/PVDC/Aluminium silver foil) of 1, 2, 3, 4 tablets.
Not all strengths, pack types and/or pack sizes may be available.
6.6 Special Precautions for Disposal
In Australia, any unused medicine or waste material should be disposed of by taking to your local pharmacy.
6.7 Physicochemical Properties
Risedronate sodium hemipentahydrate is a fine, white to off white, odourless, crystalline powder. It is soluble in water and in aqueous solutions and essentially insoluble in common organic solvents. It is also soluble in pH 7.0 potassium phosphate dibasic solution, 0.1 N sodium hydroxide solution, very slightly soluble in 0.1 N hydrochloric acid, practically insoluble in ethanol and insoluble in isopropanol.
Chemical structure.
 Chemical name: [1-hydroxy-2-(3-pyridinyl)ethylidene]bis[phosphonic acid], monosodium salt, hemipentahydrate.
Chemical name: [1-hydroxy-2-(3-pyridinyl)ethylidene]bis[phosphonic acid], monosodium salt, hemipentahydrate.
Molecular formula: C7H10NO7P2Na.2½H2O.
Molecular weight: 350.132.
CAS number.
329003-65-8.7 Medicine Schedule (Poisons Standard)
S4 - Prescription Only Medicine.
Summary Table of Changes


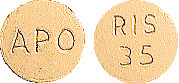

 In a 2 year study in men with osteoporosis, the overall safety and tolerability were similar between the treatment and the placebo groups. Adverse experiences were consistent with those previously observed in women.
In a 2 year study in men with osteoporosis, the overall safety and tolerability were similar between the treatment and the placebo groups. Adverse experiences were consistent with those previously observed in women.

 Three patients that received risedronate 30 mg/day experienced acute iritis in one supportive study. All three patients recovered from their events; however, in one of these patients, the event recurred during risedronate treatment and again during treatment with pamidronate. All patients were effectively treated with topical steroids.
Three patients that received risedronate 30 mg/day experienced acute iritis in one supportive study. All three patients recovered from their events; however, in one of these patients, the event recurred during risedronate treatment and again during treatment with pamidronate. All patients were effectively treated with topical steroids.
 The incidence of non-vertebral fractures in the pooled analysis (RVN and RVE) was lower in the risedronate sodium 5 mg daily group than in the placebo group for all fractures at these sites combined, as well as for the wrist, humerus, pelvis and leg separately. This difference was significant for all non-vertebral osteoporosis related fractures (p = 0.005), as well as for the humerus (p = 0.024) and pelvis (p = 0.044), while a trend was seen at the wrist (p = 0.075) (Table 6). These findings demonstrate a beneficial effect of risedronate in preventing non-vertebral, osteoporosis-related fractures.
The incidence of non-vertebral fractures in the pooled analysis (RVN and RVE) was lower in the risedronate sodium 5 mg daily group than in the placebo group for all fractures at these sites combined, as well as for the wrist, humerus, pelvis and leg separately. This difference was significant for all non-vertebral osteoporosis related fractures (p = 0.005), as well as for the humerus (p = 0.024) and pelvis (p = 0.044), while a trend was seen at the wrist (p = 0.075) (Table 6). These findings demonstrate a beneficial effect of risedronate in preventing non-vertebral, osteoporosis-related fractures.

 Very few patients in any treatment group had new fractured vertebrae at month 12 (5 mg daily: 1.5%; 35 mg once a week: 1.3%). No patient had more than one new fractured vertebra. There were no statistically significant differences in the percentage of patients with new vertebral fractures among the two treatment groups.
Very few patients in any treatment group had new fractured vertebrae at month 12 (5 mg daily: 1.5%; 35 mg once a week: 1.3%). No patient had more than one new fractured vertebra. There were no statistically significant differences in the percentage of patients with new vertebral fractures among the two treatment groups. The effect of risedronate discontinuation on bone mineral density was studied in a double-blind, placebo-controlled study in post-menopausal women with glucocorticoid dependent rheumatoid arthritis. Women were treated for two years with risedronate 2.5 mg daily, cyclic risedronate (averaged 2.5 mg risedronate per day over the 96 week active period) or placebo and then followed without treatment for one more year. Patients continued glucocorticoid treatment during the third year of the study. Risedronate discontinuation resulted in bone loss at all skeletal sites (proximal femur and lumbar spine) during the third year. The rate of bone loss, however, was similar to the placebo group indicating that bone loss was not accelerated after risedronate was discontinued. The study supports the use of continuous treatment with risedronate to prevent bone loss.
The effect of risedronate discontinuation on bone mineral density was studied in a double-blind, placebo-controlled study in post-menopausal women with glucocorticoid dependent rheumatoid arthritis. Women were treated for two years with risedronate 2.5 mg daily, cyclic risedronate (averaged 2.5 mg risedronate per day over the 96 week active period) or placebo and then followed without treatment for one more year. Patients continued glucocorticoid treatment during the third year of the study. Risedronate discontinuation resulted in bone loss at all skeletal sites (proximal femur and lumbar spine) during the third year. The rate of bone loss, however, was similar to the placebo group indicating that bone loss was not accelerated after risedronate was discontinued. The study supports the use of continuous treatment with risedronate to prevent bone loss.
 Chemical name: [1-hydroxy-2-(3-pyridinyl)ethylidene]bis[phosphonic acid], monosodium salt, hemipentahydrate.
Chemical name: [1-hydroxy-2-(3-pyridinyl)ethylidene]bis[phosphonic acid], monosodium salt, hemipentahydrate.