What is in this leaflet
This leaflet answers some of the common questions about RISPERNIA. It does not contain all of the available information. It does not take the place of talking to your doctor or pharmacist.
If you have any concerns about using RISPERNIA, ask your doctor or pharmacist. Your doctor and pharmacist have more information.
Keep this leaflet with your medicine. You may need to read it again.
What RISPERNIA is used for
RISPERNIA is used to treat symptoms of schizophrenia and other types of related psychoses. These are disorders related to thought, feeling and/or action. RISPERNIA may be taken for both sudden (acute) and long-lasting (chronic) schizophrenia.
RISPERNIA is also used to treat behavioural problems in patients with a decline in mental ability (dementia). These problems include:
- Aggression through words or action;
- Severe suspiciousness;
- Agitation; or
- Wandering.
RISPERNIA can be used to treat conduct and other disruptive behaviors such as aggression, impulsiveness and self-injury in children (over 5 years old), adolescents and adults who are intellectually disabled.
RISPERNIA helps to correct a chemical imbalance in the brain associated with these conditions.
RISPERNIA has been approved for the uses mentioned above. However, your doctor may prescribe this medicine for another use.
If you want more information, ask your doctor.
RISPERNIA is not addictive.
Before you use RISPERNIA
When you must not use it
Do not use RISPERNIA:
- If you know you are allergic to any of its ingredients (signs of allergy include skin rash, itching, shortness of breath, and/or swollen face - see the last section of this leaflet for a list of ingredients).
- If the packaging is torn or shows signs of being tampered with.
- If the tablets do not look right.
- To treat any other complaints unless your doctor says it is safe to do so.
Before you start to use it
RISPERNIA should be used with caution in some patients:
- Tell your doctor if you have or have ever had:
- heart or blood vessel diseases, including low blood pressure;
- dehydration;
- kidney or liver problems;
- Parkinson's disease;
- Dementia or Lewy body dementia;
- epilepsy;
- breast cancer;
- disease of the pituitary gland;
- disease of the blood vessels of the brain, including stroke;
- Neuroleptic Malignant Syndrome (a serious reaction to neuroleptics); or
- tardive dyskinesia (a reaction to some medicines with uncontrollable twitching or jerking movements of the arms and legs). - Tell your doctor if:
- You are pregnant or are planning to become pregnant. Your doctor will advise you whether or not you should take RISPERNIA.
- You are breast-feeding. RISPERNIA is excreted in breast milk. It is recommended that you do not breast-feed while taking RISPERNIA.
Your doctor will advise you whether or not you should take RISPERNIA. - Other medicines and alcohol:
Tell your doctor if you are taking any other medicines, including any that you buy without a prescription from your pharmacy, supermarket or health food shop. In particular, tell your doctor if you are taking:
- Sleeping tablets, tranquillisers, pain killers, antihistamines;
- Medicines to treat Parkinson’s disease or a tremor;
- Medicines to treat epilepsy;
- Medicines to treat depression, panic or anxiety disorders, obsessive-compulsive disorder;
- Medicines for your heart or blood pressure;
- Medicines to treat pre-menstrual dysphoric disorder;
- Medicines to relieve severe nausea and vomiting;
- Other medicines to treat mental illness or psychotic conditions; or
- Diuretics - medicines to treat high blood pressure and fluid build up (eg. frusemide).
In studies in elderly patients with dementia where risperidone was compared with the dummy pill (placebo), the death rate was 3.1% with the dummy pill and slightly higher at 4% with risperidone. Taking risperidone with frusemide, a medicine which is used to treat high blood pressure or to treat swelling of parts of the body caused by the build-up of too much fluid, contributed to this difference, so this combination may be harmful.
Tell your doctor if you are taking any forms of frusemide.
RISPERNIA can increase the effect of alcohol and other medicines which slow your reactions. You should not drink alcohol while taking RISPERNIA. - Elderly people
Elderly people should take less RISPERNIA than is prescribed for other adults (see How to take it).
Taking it for the first time
At the start of treatment you may have a fall in blood pressure making you feel dizzy on standing up, or your heart may beat faster. These should go away after a few days. Tell your doctor if they continue or worry you.
Using RISPERNIA
How to take it
RISPERNIA may be taken as a single dose, once a day or it may be taken in divided doses twice a day (in the morning and in the evening). The tablets should be swallowed with a glass of water. You may take RISPERNIA either with or between meals.
It is very important that you take the correct amount of RISPERNIA, but this will vary from person to person. Your doctor will adjust the number and strength of the tablets until the desired effect is obtained.
Follow your doctor's instructions carefully and do not change or stop the required dosage without consulting your doctor first.
For Schizophrenia and Related Psychoses
The usual starting dose of RISPERNIA is 1 mg twice a day. This will be gradually increased by your doctor to suit your needs. From then on, the dose can be taken once a day or twice a day according to your doctor's instructions. For long-term treatment, your doctor will determine the dose most suitable for you.
Important note: never take more tablets than your doctor tells you to take. The effects of high doses are not yet known. Please double check with your doctor if your doctor prescribes more than 5 milligrams twice a day.
RISPERNIA cannot be recommended for use in children with schizophrenia under 15 years at the present time as there is little experience with the product in this group.
For Elderly Patients with Schizophrenia or Related Psychoses
For older patients a starting dose of 0.5 mg (half a 1 mg tablet) twice a day (in the morning and in the evening) is usual. The dose may be increased by 0.5 mg twice daily to 1 to 2 mg twice a day (in the morning and in the evening).
Patients with Impaired Kidney and Liver Function
If you have kidney or liver disease, a starting dose of 0.5 mg (half a 1 mg tablet) twice a day (in the morning and in the evening) is usual. The dose may be increased by 0.5 mg twice daily to 1 to 2 mg twice a day (in the morning and in the evening).
For Behavioural Problems in People with Dementia
The usual starting dose is 0.25 mg twice daily. This may be gradually increased by your doctor to suit your needs.
From then on, the dose can be taken once a day or twice a day according to your doctor's instructions. For long-term treatment, 1 mg daily is the usual dose but your doctor will determine the dose most suitable for you.
For Disruptive Behaviour Disorders in Adults and Children
For people who weigh 50 kg or more, the usual starting dose is 0.5 mg (half a 1 mg tablet) once a day. The dose may be increased by 0.5 mg once every two days, to the usual dose of 0.5 to 1.5 mg once a day.
For people who weigh less than 50 kg, the usual starting dose is 0.25 mg once a day. The dose may be increased by 0.25 mg once every two days, to the usual dose of 0.25 to 0.75 mg once a day.
Your doctor will advise you on how much RISPERNIA you need.
RISPERNIA cannot be recommended for use in children with disruptive behaviour disorders under 5 years at the present time, as there is little experience with the product in this group.
If you forget to take RISPERNIA
If you forget to take RISPERNIA, take the missed dose as soon as you remember instead of your next dose. Then go back to taking it as you would normally.
Do not take a double dose to make up for the one you missed.
If you forget to take RISPERNIA for 5 days or more, tell your doctor before starting your medicine again.
If you have problems remembering when to take your medicine, ask your pharmacist for some hints.
If you take too much (Overdose)
Immediately telephone your doctor or pharmacist or the Poisons Information Centre (telephone Australia 13 11 26), or go to Accident and Emergency at your nearest hospital, if you think that you or anyone else may have taken too much RISPERNIA. Do this even if there are no signs of discomfort or poisoning.
Signs of overdose may include drowsiness, sleepiness, excessive trembling, excessive muscle stiffness, increased heart rate, very low blood pressure causing fainting or unconsciousness.
While you are using RISPERNIA
Things you must do
- Always follow your doctor's instructions carefully, and seek your doctor's advice before changing or stopping treatment. Your doctor will be happy to discuss any questions you may have with your treatment.
- Try to eat a moderate diet. RISPERNIA can cause weight gain.
- Pre-menopausal women should tell their doctor if they do not have a period for more than six months while taking RISPERNIA.
Things to be careful of
- Do not drink alcohol. RISPERNIA can increase the effects of alcohol.
- Ask your doctor before taking any other medicines. RISPERNIA can increase the effects of medicines which slow your reactions. Always ask your doctor or pharmacist before taking other medicines. These include herbal treatments and those bought in a pharmacy or supermarket.
- Be careful driving or operating machinery until you know how RISPERNIA affects you. RISPERNIA may cause dizziness or light-headedness in some people, especially after the first dose. Make sure you know how you react to RISPERNIA before you drive a car, operate machinery, or do anything else that could be dangerous if you are dizzy.
- Avoid excessive eating as there is a possibility of weight gain when taking RISPERNIA.
Side Effects
All medicines can have side effects. Sometimes they are serious, but most of the time they are not. RISPERNIA is generally well-tolerated and side effects are often hard to distinguish from the disease symptoms. You may need medical treatment if you get some of the side effects.
Tell your doctor as soon as possible if you do not feel well while you are using RISPERNIA.
Below is a list of possible side effects you could get while taking RISPERNIA:
- sleeplessness;
- agitation;
- anxiety;
- headache;
- trembling;
- excessive saliva;
- muscle stiffness;
- restlessness in the legs;
- fall in blood pressure, particularly on standing. This will be apparent to you as light-headedness or dizziness that passes after a few seconds or after sitting down again; or
- fast heart rate.
Although these effects are generally not harmful, contact your doctor if they bother you too much.
The following may occur less often:
- drowsiness, tiredness, difficult in concentrating, somnolence, usually mild and short lasting may occur more often in children than in adults;
- blurred vision;
- dizziness;
- indigestion, nausea, abdominal pain, constipation;
- sexual function disturbances;
- some loss of bladder control;
- blocked nose;
- weight gain; or
- excessive thirst.
During a long treatment, twitching of the tongue, face, mouth and jaws can occur.
Should this happen contact your doctor:
- In the early stages of treatment, in some people, blood pressure may decrease slightly and the heart beat increase resulting in dizziness. This usually goes away after a few days. (See Taking it for the first time).
- After taking RISPERNIA for a long time, some women may experience breast enlargement or get a discharge from the breasts. They may also experience irregular or heavy periods or absence of their periods. In men, breasts may enlarge slightly.
The following may occur rarely:
- Oversensitivity (allergy). (See when you must not use it).
- In extremely rare cases, significant changes in body temperature may occur. This rise or fall in temperature is caused by a combination of several factors such as extreme cold or heat. Call your doctor if this happens.
- In elderly patients with dementia, sudden weakness or numbness of the face, arms or legs, especially on one side, instances of slurred speech and stroke have been seen. If any of these should occur, even if for a short period of time, seek medical attention right away.
- In very rare cases, high blood sugar has been reported. The symptoms of high blood sugar may be the need to urinate more often or feeling thirsty all the time. Contact your doctor if you experience any of these symptoms.
IMPORTANT: If you experience high fever, stiff muscles, fast breathing, abnormal sweating or decreased mental alertness, contact your doctor immediately. Your body may not be reacting properly to the medicine.
Do not hesitate to report any other side effects to your doctor or pharmacist.
After using RISPERNIA
Storage
Keep RISPERNIA tablets in a dry place where the temperature stays below 25°C.
Do not store RISPERNIA tablets or any medicines in the bathroom or near a sink. Heat and dampness can destroy some medicines.
Keep the tablets where young children cannot reach them. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Do not use RISPERNIA beyond the date (month and year) printed on the pack after the letters "EXP", even if it has been stored properly. Medicines cannot be stored indefinitely.
Do not use RISPERNIA if the appearance of the tablets has changed.
Disposal
Once you have finished using RISPERNIA, ask your pharmacist what to do with any unused medicine.
Product Description
What it looks like
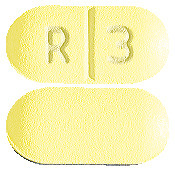
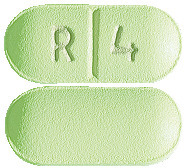
You can identify RISPERNIA tablets by their colour and shape. This is important because there are several strengths of RISPERNIA, each containing a different amount of risperidone:
- 0.5 mg red, round tablets marked “R” on one side and scored on the other.
- 1 mg white, oblong scored tablets marked “R” “1” on the scored side.
- 2 mg orange, oblong scored tablets marked “R” “2” on the scored side.
- 3 mg yellow, oblong scored tablets marked “R” “3” on the scored side.
- 4 mg green, oblong scored tablets marked “R” “4” on the scored side.
- 6 mg yellow, oblong scored tablets marked “R” “6” on the scored side.
Ingredients
Active ingredient:
- Risperidone
Excipients:
- Lactose;
- Microcrystalline cellulose;
- Maize starch;
- Colloidal anhydrous silica;
- Magnesium stearate;
- Hypromellose;
- Titanium dioxide;
- Macrogol 400;
- Iron oxide red - 0.5 mg, 2 mg;
- Quinoline yellow - 2 mg, 3 mg, 4 mg, 6 mg;
- Indigotin - 4 mg.
RISPERNIA tablets are available in blister and bottle packs of 60 tablets.
Distributor
Eris Pharmaceuticals (Australia) Pty Ltd
6 Eastern Road
South Melbourne Vic 3205
www.eris-pharma.com.au

Australian Registration Numbers:
- Blister packs:
0.5 mg AUST R 127916
1 mg AUST R 127919
2 mg AUST R 127921
3 mg AUST R 127923
4 mg AUST R 127925
6 mg AUST R 127927 - Bottle packs:
0.5 mg AUST R 127918
1 mg AUST R 127920
2 mg AUST R 127922
3 mg AUST R 127924
4 mg AUST R 127926
6 mg AUST R 127930
This leaflet was prepared in August 2013.
Published by MIMS January 2015