What is in this leaflet
This leaflet answers some common questions about Ritalin LA (long-acting) capsules.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
The information in this leaflet was last updated on the date listed on the final page. More recent information on the medicine may be available.
You should ensure that you speak to your pharmacist or doctor to obtain the most up to date information on the medicine. You can also download the most up to date leaflet from www.novartis.com.au. Those updates may contain important information about the medicine and its use of which you should be aware.
All medicines have risks and benefits. Your doctor has weighed the risks of you or your child taking this medicine against the benefits they expect it will provide.
If you have any concerns about this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What Ritalin LA is used for
Ritalin LA contain the active ingredient methylphenidate hydrochloride. Methylphenidate hydrochloride is a central nervous system stimulant.
Ritalin LA (long-acting) capsules are used to treat Attention Deficit Hyperactivity Disorder (ADHD).
Ritalin LA is a stimulant that increases attention and decreases impulsiveness and hyperactivity in patients with ADHD. Ritalin LA is thought to work by regulating specific chemicals in the brain that affect behaviour. It helps to focus attention, shut out distraction and allows impulsive people to think before they act. If successful, it will enhance an inattentive person's natural ability.
Ritalin LA should be given as part of a total treatment program for ADHD that may include other measures (psychological, educational and social). It is not intended for use in patients who have symptoms due to environmental factors and/or other primary psychiatric disorders, including psychosis.
Ask your doctor if you have any questions about why this medicine has been prescribed for you or your child. Your doctor may have prescribed it for another purpose.
Ritalin LA, like all medicines containing central nervous system stimulants, will be given to you only under close medical supervision and after diagnosis.
This medicine is only available with a doctor's prescription and your doctor has special permission to prescribe it.
There is not enough information to recommend Ritalin LA use in children under 6 years old.
Before you take Ritalin LA
When you must not take it
Do not take Ritalin LA if you are allergic (hypersensitive) to methylphenidate (the active ingredient) or to any of the other ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue or other parts of the body; rash, itching or hives on the skin.
Do not take Ritalin LA if you have any of the following medical conditions:
- periods of severe anxiety, tension or agitation
- Tourette's syndrome (a condition with uncontrolled speech and body movements or tics) or you have a family history of this disorder
- tics (muscle twitching which is usually in the face or shoulders) or if your brothers or sisters have tics
- increased pressure in the eye (glaucoma)
- an overactive thyroid (hyperthyroidism) or other thyroid problems
- heart problems such as heart attack, irregular heartbeat, chest pain (angina), heart failure, heart disease or if you were born with a heart problem
- very high blood pressure (hypertension) or narrowing of the blood vessels (arterial occlusive disease, which can cause pain in the arms and legs)
- severe depression or other mental illness
- a tumour of the adrenal gland, which sits near the kidney (pheochromocytoma)
If you are not sure whether any of the above medical conditions apply to you, check with your doctor.
Do not take Ritalin LA if you are taking a medicine called a monoamine oxidase inhibitor (MAOI) or have been taking it within the past 14 days. Taking Ritalin LA together with MAOI medicines may cause a serious reaction with a sudden increase in body temperature, extremely high blood pressure and severe convulsions.
Ask your doctor or pharmacist if you are not sure if you have been taking one of these medicines.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. In that case, return it to your pharmacist.
Before you start to take it
Tell your doctor if you are allergic to any other medicines, foods, dyes or preservatives. Your doctor will want to know if you are prone to allergies.
Tell your doctor if you have any of the following medical conditions or behaviours:
- any heart defects (e.g. structural cardiac abnormality)
- a family history of sudden death and /or irregular heart beat
- hardening of the arteries
- any other current or previous heart problems
- any disorders of the blood vessels in the brain, e.g. weakening of the blood vessel (aneurysm), stroke, or inflammation of blood vessels (vasculitis)
- severe depression, bipolar disorder or other mental illness
- epilepsy (seizures, convulsions, or fits)
- high blood pressure
- history of alcohol or drug abuse or dependence
- acute mental disorders that cause abnormal thinking and perceptions (psychosis) or feeling unusually excited, over-active and un-inhibited (acute mania) - your doctor will have told you if you have this
- psychotic symptoms such as seeing or feeling things that are not really there (hallucinations)
- aggressive behaviour
- suicidal thoughts or behaviour
- fingers and toes feeling numb, tingling and changing colour (from white to blue, then red) when cold ('Raynaud's phenomenon').
- have or develop eye problems, including increased pressure in your eye, glaucoma, or problems with your close-up vision (farsightedness).
Your doctor may want to take special precautions if you have any of the above conditions.
Tell your doctor if you are pregnant or breast-feeding. Ask your doctor about the risks and benefits of taking Ritalin LA in this case. Ritalin LA is not to be used during pregnancy unless specifically prescribed by your doctor. This medicine may affect your developing baby if you take it while you are pregnant.
Do not breast-feed during treatment with Ritalin LA. The active ingredient in Ritalin LA can pass into the breast milk.
Taking other medicines
Tell your doctor if you are taking any other medicines, including medicines that you buy without a prescription from a pharmacy, supermarket or health food shop.
Some medicines and Ritalin LA may interfere with each other. It may be necessary to change the dose or in some cases to stop one of the medicines.
Some of these medicines include:
- medicines that increase blood pressure
- alpha 2 agonists like clonidine (used to treat high blood pressure)
- medicines used to treat depression, such as tricyclic antidepressants and monoamine oxidase inhibitors (MAO inhibitors)
- some anticonvulsants (medicines used to treat epilepsy or fits)
- oral anticoagulants or warfarin (medicines used to prevent blood clots)
- phenylbutazone (used to treat pain or fever)
- guanethidine
- anaesthetics
- medicines that influence the level of dopamine in the body (dopaminergic medicines used to treat Parkinson's disease or psychosis)
- medicines that raise the level of serotonin in the body (serotonergic medicines, for example those used to treat depression like sertraline and venlafaxine).
You or your child may need to take different amounts of your medicines or you or your child may need to take different medicines. Your doctor and pharmacist have more information.
If you have not told your doctor about any of these things, tell him/her before you or your child start taking this medicine.
How to take Ritalin LA
Your doctor will decide on the most suitable dosage according to the individual patient's medical need and response.
Follow all directions given to you by your doctor and pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the label, ask your doctor or pharmacist for help.
How much to take
Ritalin LA is available in long-acting capsules containing 10 mg, 20 mg, 30 mg, 40 mg or 60 mg of methylphenidate hydrochloride as the active ingredient.
Your doctor will usually start with a low dose and, if necessary, increase it gradually at weekly intervals up to 20 mg to 40 mg each day.
Do not exceed the maximum recommended dose.
In children, the maximum recommended daily dose is 60 mg. In adults, the maximum recommended daily dose is 80 mg.
How to take it
You can take Ritalin LA with or without food. Swallow the capsules whole as a single dose in the morning with a full glass of water. Do not crush or chew the capsules.
If you have trouble swallowing the capsules, you can carefully open them and sprinkle the contents (little beads) over a small amount of soft food (e.g. applesauce). The soft food should not be warm as this could affect the special properties of the beads.
Immediately eat all of the soft food and Ritalin LA mixture. Do not store any of the soft food that has been mixed with Ritalin LA for future use.
If the capsules upset your stomach, you can take them with food, but always take them in the same way (e.g. always with food or always without food). That way the effect will always be the same.
How long to take it
Continue taking Ritalin LA for as long as your doctor tells you.
This medicine helps to control your symptoms but does not cure your condition. Your doctor will check your progress to make sure the medicine is working and will discuss with you how long your treatment should continue.
Treatment for ADHD varies in length from patient to patient. During treatment for ADHD, your doctor may stop Ritalin LA every so often (e.g. over weekends or school holidays) to see whether it is still needed. Breaks from treatment also help to prevent a slow-down in growth that sometimes happens when children take this medicine for a long time.
If you forget to take it
If you forget to take a dose of Ritalin LA capsules and you remember before mid-day, take the dose as soon as you remember. Then go back to your usual schedule on the following day.
If you do not remember before mid-day, miss your dose of Ritalin LA for that day and wait until the following morning to take your next dose.
Do not take a double dose to make up for the one that you missed. Your chance of an unwanted side effect may be increased if you do.
If you have trouble remembering when to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26), or go to Accident and Emergency at your nearest hospital if you think that you or anyone else may have taken too much Ritalin LA. Do this even if there are no signs of discomfort or poisoning. Keep the telephone numbers for these places handy.
Some of the symptoms of an overdose may include vomiting, agitation, headache, tremors, muscle twitching, irregular heart beat, flushing, fever, sweating, dilated pupils, breathing problems, confusion, seizures and muscle spasms accompanied by fever and red-brown urine.
While you are taking Ritalin LA
Things you must do
Take Ritalin LA exactly as your doctor has prescribed. Like all stimulants, this medicine may become habit-forming and can be abused by some people. If you take it correctly as instructed by your doctor, abuse or dependence should not be a problem, either now or later in life.
Be sure to keep all of your doctor's appointments so that your or your child's progress can be checked. Your doctor will want to check your or your child's blood pressure, height, weight and do blood tests from time to time to prevent unwanted side effects from happening.
If your child is not growing or gaining height or weight as expected, treatment with Ritalin LA may need to be interrupted.
If you become pregnant while taking Ritalin LA, tell your doctor. Your doctor can discuss with you the risks and benefits of taking it while you are pregnant.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking Ritalin LA.
Tell any doctor, dentist or pharmacist who treats you that you are taking Ritalin LA.
Things you must not do
Do not drink alcohol whilst you are taking Ritalin LA. Remember that some foods and medicines contain alcohol. Drinking alcohol is not recommended because it can worsen some of the unwanted effects of this medicine, such as dizziness and drowsiness.
Do not stop your treatment without first checking with your doctor. If you suddenly stop taking this medicine, your condition may reappear or you may get unwanted effects such as depression. To prevent this, your doctor may want to gradually reduce the amount of medicine you take each day before stopping it completely.
You will need medical supervision after having interrupted the treatment.
Do not change the dose without talking to the doctor. If you have the impression that the effect of Ritalin LA is too strong or too weak, talk to the doctor.
Do not take this medicine to treat any other complaints unless your doctor tells you to.
Do not give it to anyone else, even if their condition seems similar to yours.
Things to be careful of
Be careful driving, operating machinery or doing jobs that require you to be alert while you are taking Ritalin LA until you know how it affects you. This medicine may cause hallucinations, dizziness, drowsiness, blurred vision or other central nervous system side effects which can affect concentration in some people. If you have any of these symptoms, do not drive, use machines or do anything else that needs quick reactions or could be dangerous.
Ritalin LA may give a false positive result when testing for drug use. This includes testing used in sport.
Some children taking Ritalin LA for a long time may have slower than normal growth, but they usually catch up once the treatment is stopped.
In some patients Ritalin LA may cause stomach upset, loss of appetite and difficulty sleeping, especially at the start of treatment. Your doctor can usually help to reduce these symptoms by lowering the dose of Ritalin LA or changing the times when the tablets are taken.
Tell your doctor if you are going to have an operation. There is a chance of a sudden rise in blood pressure during the operation if an anaesthetic is used. Your doctor will advise if you/your child should take Ritalin LA on the day of the operation.
If you experience abnormally sustained or frequent and painful erections of the penis on Ritalin LA treatment or after treatment discontinuation, you may need urgent medical treatment. This can occur in any age group.
If this occurs, tell your doctor immediately.
If taking Ritalin LA with medicines that raise the level of serotonin in the body (serotonergic medicines, e.g. sertraline and venlafaxine used to treat depression) and you experience a combination of the following symptoms: restlessness, tremor, sudden muscle contractions, abnormal high temperature, nausea and vomiting, stop treatment with Ritalin LA and these medicines and tell your doctor immediately.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Ritalin LA.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Do not be alarmed by these lists of possible side effects. You may not experience any of them. Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you notice any of the following and they worry you:
- excessive emotional distress or excitement
- nervousness
- feeling anxious, agitated
- feeling jittery
- feeling depressed
- feeling aggressive
- unusually active, depressed mood
- uncontrolled speech and body movements (Tourette's syndrome)
- troubled sleep or restlessness, difficulty falling asleep, sleep disturbance, sleepiness
- nausea (feeling sick)
- dry mouth
- vomiting, stomach pain, upset stomach, indigestion, toothache
- loss of appetite, decreased weight
- excessive sweating
- loss of weight and slower growth in children
- sore throat and runny nose
- headache
- cough
- dizziness
- blurred vision or problems focussing your eyes
- muscle cramps
- fever
- hair loss
- abnormal heart rhythm
- palpitations
- involuntary shaking of the body (sign of tremor)
- skin rash, itchy rash and hives
- joint pain
- excessive teeth grinding
- spasm of the jaw muscles that makes it difficult to open the mouth
- stuttering
- bedwetting in children during the night
Tell your doctor immediately or go to Accident and Emergency at your nearest hospital if you notice any of the following:
- swelling of the face, lips or tongue (severe allergic reaction)
- sudden high fever, severe convulsions
- severe headache or confusion, weakness or paralysis of limbs or face, difficulty speaking
- fast heartbeat, chest pain
- uncontrollable twitching, jerking
- bruising
- muscle twitching or tics
- a sore throat and fever or chills
- uncontrollable writing movements of the limb, face and/ or trunk
- seeing or feeling things that are not really there (hallucination)
- fits (seizures, convulsions, epilepsy)
- skin blisters or itching
- red blotches on the skin
- prolonged erection, causing discomfort of the penis (sign of priapism).
- thoughts or attempts of killing yourself (suicidal ideation or attempt (including completed suicide)).
- fingers and toes feeling numb, feeling cold, tingling and changing colour (from white to blue, then red) when cold (Raynaud’s phenomenon, peripheral coldness)
- abnormal decrease in the levels of all type of blood cells. This is associated with anaemia, decreased platelets, and decreased white blood cells. This can lead to fatigue, increased risk of bleeding, and/or increased risk of infection. This may be serious or life threatening (pancytopenia).
The above side effects may be serious. You may need urgent medical attention.
Other possible side effects with unknown frequency
- swelling of the ears (a symptom of allergic reaction)
- stuttering, feeling irritated, mood changes, abnormal behaviour or thinking, anger, excessive awareness of surroundings, feeling over-active and uninhibited (mania), feeling disorientated, changes in sex drive, lack of feeling or emotion, doing things over and over again, being obsessed with one thing, confusion, addiction
- temporary muscle weakness, loss of skin sensation or other functions of the body due to a temporary lack of blood supply to the brain (reversible ischemic neurological deficit), migraine
- double vision, dilated pupils, trouble seeing
- stopped heartbeat, heart attack
- sore throat, shortness of breath
- diarrhoea, constipation
- swelling of face and throat, redness of the skin, large red blotches on the skin appearing within a few hours of taking the medicine
- muscle pain, muscle twitching;
- inability to develop or maintain an erection, swelling of the breasts in men
- tiredness
- increased eye pressure (increased intraocular pressure)
- nose bleed
Additional side effects that occurred with other medicines containing the same drug substance of Ritalin (frequency unknown):
- blood in the urine
- chest pain, sudden death of heart origin
- abnormal sounds from heart.
Tell your doctor if any of these side effects occur or if you notice anything else that is making you feel unwell. Some people may have other side effects not yet known or mentioned in this leaflet.
After taking Ritalin LA
Storage
- Keep your medicine in the original container until it is time to take a dose.
- Store it in a cool dry place.
- Do not store Ritalin LA or any other medicine in the bathroom or near a sink.
- Do not leave it in the car or on window sills.
Keep this medicine where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine you have left over.
Product description
What it looks like
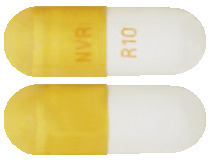
Ritalin LA 10 mg capsules are white to off-white beads in a light brown and white capsule with imprint NVR and R10 in tan-coloured ink; bottles of 30 capsules.
Ritalin LA 20 mg capsules are white to off-white beads in a white capsule with imprint NVR and R20 in tan-coloured ink; bottles of 30 capsules.
Ritalin LA 30 mg capsules are white to off-white beads in a yellow capsule with imprint NVR and R30 in tan-coloured ink; bottles of 30 capsules.
Ritalin LA 40 mg capsules are white to off-white beads in a light brown capsule with imprint NVR and R40 in tan-coloured ink; bottles of 30 capsules.
Ritalin LA 60 mg capsules are white to off-white beads in a light brown opaque cap and yellow opaque body hard gelatin capsule, with imprint NVR on cap and R60 on body in tan-coloured ink; bottles of 30 capsules.
Ingredients
Ritalin LA capsules contain 10 mg, 20 mg, 30 mg, 40 mg or 60 mg of methylphenidate hydrochloride as the active ingredient. They also contain:
- sugar spheres (sucrose and maize starch)
- ammonio methacrylate copolymer
- methacrylic acid copolymer
- purified talc (E553b)
- triethyl citrate (E1505)
- macrogol 6000
- gelatin
- titanium dioxide (E171)
- iron oxide yellow CI77492 (E172) (30 mg,40 mg and 60 mg capsules only)
- iron oxide black CI77499 (E172) (40 mg and 60 mg capsules only)
- iron oxide red CI77491 (E172) (40 mg and 60 mg capsules only)
- TekPrint SW-8010
Ritalin LA capsules do not contain gluten.
Sponsor
Ritalin LA capsules are supplied in Australia by:
NOVARTIS Pharmaceuticals Australia Pty Limited
ABN 18 004 244 160
54 Waterloo Road
Macquarie Park NSW 2113
Telephone: 1 800 671 203
Web site: www.novartis.com.au
® = Registered Trademark
This leaflet was prepared in January 2024.
Australian Registration Number
Ritalin LA 10 mg: AUST R 160228
Ritalin LA 20 mg: AUST R 82957
Ritalin LA 30 mg: AUST R 82958
Ritalin LA 40 mg: AUST R 82959
Ritalin LA 60 mg: AUST R 236251
Internal Document Code:
(rtlLA300124c) based on (rtl300124i)
Published by MIMS March 2024




 For other methylphenidate regimens, clinical judgement should be used when selecting the starting dose.
For other methylphenidate regimens, clinical judgement should be used when selecting the starting dose.


 The AE profile seen in the maintenance period was similar to that observed in the core study. No unexpected SAEs or AEs were observed in this extension study and the commonly observed AEs were expected and driven by the pharmacologic activity.
The AE profile seen in the maintenance period was similar to that observed in the core study. No unexpected SAEs or AEs were observed in this extension study and the commonly observed AEs were expected and driven by the pharmacologic activity. Very rare reports of poorly documented neuroleptic malignant syndrome (NMS) have been received. In most of these reports, patients were also receiving other medications. It is uncertain what role Ritalin played in these cases.
Very rare reports of poorly documented neuroleptic malignant syndrome (NMS) have been received. In most of these reports, patients were also receiving other medications. It is uncertain what role Ritalin played in these cases.

 Significant clinical efficacy was demonstrated in all three Ritalin LA dose levels using physician rated scales [Clinical Global Impression-Improvement (CGI-I) and Clinical Global Improvement-Severity (CGI-S)], self rated scales [Adult Self-Rating Scale (ASRS)] and observer rated scales [Conners' Adult ADHD Rating Scale Observer Short Version (CAARS O:S)]. The results were consistently in favour of Ritalin LA over placebo across all assessments in period 1.
Significant clinical efficacy was demonstrated in all three Ritalin LA dose levels using physician rated scales [Clinical Global Impression-Improvement (CGI-I) and Clinical Global Improvement-Severity (CGI-S)], self rated scales [Adult Self-Rating Scale (ASRS)] and observer rated scales [Conners' Adult ADHD Rating Scale Observer Short Version (CAARS O:S)]. The results were consistently in favour of Ritalin LA over placebo across all assessments in period 1. Patients who entered Period 3 had completed a total of between 5-14 weeks of Ritalin LA treatment in Periods 1 and 2. Patients then assigned to placebo in Period 3 did not experience increased signs of withdrawal and rebound compared to patients who continued on Ritalin LA treatment. The study performed in adults did not suggest any difference in efficacy or safety amongst gender subgroups (see Section 4.2 Dose and Method of Administration).
Patients who entered Period 3 had completed a total of between 5-14 weeks of Ritalin LA treatment in Periods 1 and 2. Patients then assigned to placebo in Period 3 did not experience increased signs of withdrawal and rebound compared to patients who continued on Ritalin LA treatment. The study performed in adults did not suggest any difference in efficacy or safety amongst gender subgroups (see Section 4.2 Dose and Method of Administration).

