1 Name of Medicine
Asciminib hydrochloride.
2 Qualitative and Quantitative Composition
Each 20 mg film-coated tablet contains 21.62 mg asciminib hydrochloride, which is equivalent to 20 mg asciminib.
Each 40 mg film-coated tablet contains 43.24 mg asciminib hydrochloride, which is equivalent to 40 mg asciminib.
Scemblix tablets contain sugars as lactose and soyabean products.
For the full list of excipients, see Section 6.1 List of Excipients.
3 Pharmaceutical Form
20 mg film-coated tablets.
Pale yellow, round, biconvex, film-coated tablets with beveled edges, approximately 6.2 mm diameter, unscored, debossed with "Novartis" logo on one side and "20" on the other side.
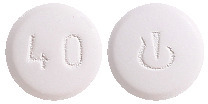
40 mg film-coated tablets.
Violet white, round, biconvex, film-coated tablets with beveled edges, approximately 8.2 mm diameter, unscored, debossed with "Novartis" logo on one side and "40" on the other side.4.1 Therapeutic Indications
Scemblix is indicated for the treatment of patients 18 years of age and above with:
Newly diagnosed Philadelphia chromosome-positive chronic myeloid leukaemia (Ph+ CML) in chronic phase (CP).
Ph+ CML in CP previously treated with two or more tyrosine kinase inhibitors.
Ph+ CML in CP with the T315I mutation.
(See Section 5.1, Clinical trials).
4.2 Dose and Method of Administration
Treatment with Scemblix should be initiated by a physician experienced in the use of anticancer therapies and should be continued as long as clinical benefit is observed or until unacceptable toxicity occurs.
Dose regimen.
Ph+ CML-CP.
The recommended total daily dose of Scemblix is 80 mg.
Scemblix can be taken orally either as 80 mg once daily at approximately the same time each day, or as 40 mg twice daily at approximately 12-hour intervals.
Patients changing from 40 mg twice daily to 80 mg once daily should start taking Scemblix once daily approximately 12 hours after the last twice-daily dose, and then continue at 80 mg once daily.
Patients changing from 80 mg once daily to 40 mg twice daily should start taking Scemblix twice daily approximately 24 hours after the last once-daily dose and then continue at 40 mg twice daily at approximately 12-hour intervals.
Any change in the dosage regimen is at the prescriber's discretion, as necessary for the management of the patient.
Ph+ CML CP harbouring the T315I mutation.
The recommended dose of Scemblix is 200 mg taken orally twice daily at approximately 12 hour intervals.
Missed dose.
Once-daily dosage regimen.
If a Scemblix dose is missed by more than approximately 12 hours, it should be skipped and the next dose should be taken as scheduled.
Twice-daily dosage regimen.
If a Scemblix dose is missed by more than approximately 6 hours, it should be skipped and the next dose should be taken as scheduled.
Dose modifications.
Ph+ CML.
For the management of adverse drug reactions, Scemblix dose can be reduced based on individual safety and tolerability, as described in Table 1. If adverse drug reactions are effectively managed, Scemblix may be resumed as described in Table 1.
Scemblix should be permanently discontinued in patients unable to tolerate a total daily dose of 40 mg.
Ph+ CML-CP harbouring the T315I mutation.
For the management of adverse drug reactions, Scemblix dose can be reduced based on individual safety and tolerability, as described in Table 1. If adverse drug reactions are effectively managed, Scemblix may be resumed as described in Table 1.
Scemblix should be permanently discontinued in patients unable to tolerate a dose of 160 mg twice daily.
 The recommended dosage modification for the management of selected adverse drug reactions is shown in Table 2.
The recommended dosage modification for the management of selected adverse drug reactions is shown in Table 2.

Method of administration.
Scemblix should be taken orally without food. Food consumption should be avoided for at least 2 hours before and 1 hour after taking Scemblix (see Section 4.5 Interactions with Other Medicines and Other Forms of Interactions; Section 5.2 Pharmacokinetic Properties).
Scemblix film-coated tablets should be swallowed whole and should not be broken, crushed or chewed.
Special populations.
Hepatic impairment.
No dose adjustment is required in patients with mild, moderate or severe hepatic impairment receiving Scemblix. Caution should be exercised in patients with severe hepatic impairment receiving Scemblix 200 mg twice daily dose (see Section 5.2 Pharmacokinetic Properties).
Renal impairment.
No dose adjustment is required in patients with mild, moderate or severe renal impairment receiving Scemblix. Caution should be exercised in patients with severe renal impairment receiving Scemblix 200 mg twice daily dose (see Section 5.2 Pharmacokinetic Properties).
Paediatric patients (below 18 years).
The safety and efficacy of Scemblix in paediatric patients (below 18 years) has not been established.
Elderly patients (65 years of age or above).
No dose adjustment is required in patients 65 years of age or above.4.3 Contraindications
Known hypersensitivity to the active substance asciminib or to any of the excipients listed in Section 6.1.
4.4 Special Warnings and Precautions for Use
Identified precautions.
Myelosuppression.
Thrombocytopaenia, neutropaenia and anaemia occurred in patients receiving Scemblix. Severe (NCI CTCAE grade 3 or 4) thrombocytopaenia and neutropaenia reactions were reported during treatment with Scemblix (see Section 4.8 Adverse Effects (Undesirable Effects)).
Myelosuppression was generally reversible and managed by temporarily withholding Scemblix. Complete blood counts should be performed every two weeks for the first 3 months of treatment and monthly thereafter, or as clinically indicated. Patients should be monitored for signs and symptoms of myelosuppression.
Based on the severity of thrombocytopaenia and/or neutropaenia, the Scemblix dose should be reduced, temporarily withheld or permanently discontinued as described in Table 2 (see Section 4.2 Dose and Method of Administration).
Pancreatic toxicity.
Clinical pancreatitis events occurred in 11 of 556 (2%) patients receiving Scemblix, with grade 3 reactions occurring in 6 (1.1%) patients. Scemblix was permanently discontinued in 3 (0.5%) patients, while it was temporarily withheld in 6 (1.1%) patients due to pancreatitis. Asymptomatic elevation of serum lipase and amylase occurred in 107 of 556 (19.2%) patients receiving Scemblix (see Table 3, "pancreatic enzymes increased"), with grade 3 and 4 reactions occurring in 41 (7.4%) and 11 (2%) patients, respectively. Scemblix was permanently discontinued in 11 (2%) patients due to the symptomatic elevation of serum lipase and amylase.
Serum lipase and amylase levels should be assessed monthly during treatment with Scemblix, or as clinically indicated. Patients should be monitored for signs and symptoms of pancreatic toxicity. More frequent monitoring should be performed in patients with a history of pancreatitis. If serum lipase and amylase elevation are accompanied by abdominal symptoms, treatment should be temporarily withheld and appropriate diagnostic tests should be considered to exclude pancreatitis (see Section 4.2 Dose and Method of Administration).
Based on the severity of serum lipase and amylase elevation, the Scemblix dose should be reduced, temporarily withheld or permanently discontinued as described in Table 2 (see Section 4.2 Dose and Method Administration).
QT prolongation.
Electrocardiogram QT prolongation occurred in 5 of 556 (0.9%) patients receiving Scemblix (see Section 4.8 Adverse Effects (Undesirable Effects)). In the ASCEMBL clinical study, one patient had a prolonged QTcF greater than 500 ms together with more than 60 ms QTcF increase from baseline and one patient had prolonged QTcF with more than 60 ms QTcF increase from baseline.
It is recommended that an electrocardiogram is performed prior to the start of treatment with Scemblix and monitored during treatment as clinically indicated. Hypokalaemia and hypomagnesaemia should be corrected prior to Scemblix administration and monitored during treatment as clinically indicated.
Caution should be exercised when administering Scemblix at a total daily dose of 80 mg concomitantly with medicinal products with a known risk of torsades de pointes. Coadministration of Scemblix at 200 mg twice daily concomitantly with medicinal products with a known risk of torsades de pointes should be avoided (see Section 4.5 Interactions with Other Medicines and Other Forms of Interactions; Section 5.1 Pharmacodynamic Properties).
Hypertension.
Hypertension occurred in 88 of 556 (15.8%) patients receiving Scemblix, with grade 3 and 4 reactions reported in 47 (8.5%) and 1 (0.2%) patients, respectively. Among the patients with hypertension ≥ grade 3, the median time to first occurrence of reactions was 21.29 weeks (range: 0.14 to 365 weeks). Scemblix was temporarily withheld in 5 (0.9%) patients due to hypertension.
Hypertension should be monitored and managed using standard antihypertensive therapy during treatment with Scemblix as clinically indicated.
For grade 3 or higher hypertension, temporarily withhold, reduce dose, or permanently discontinue Scemblix depending on persistence of hypertension (see Section 4.2 Dose and Method of Administration).
Hypersensitivity.
Hypersensitivity events occurred in 169 of 556 (30.4%) patients receiving Scemblix, with ≥ grade 3 events reported in 8 (1.4%) patients. Patients should be monitored for signs and symptoms of hypersensitivity and appropriate treatment should be initiated as clinically indicated.
Hepatitis B reactivation.
Reactivation of hepatitis B virus (HBV) has occurred in patients who are chronic carriers of this virus following administration of other BCR::ABL1 tyrosine kinase inhibitors (TKIs). Patients should be tested for HBV infection before the start of treatment with Scemblix. HBV carriers who require treatment with Scemblix should be closely monitored for signs and symptoms of active HBV infection throughout therapy and for several months following termination of therapy.
Embryo-fetal toxicity.
Based on findings from animal studies, Scemblix can cause fetal harm when administered to a pregnant woman. Pregnant women and females of reproductive potential should be advised of the potential risk to a fetus if Scemblix is used during pregnancy or if the patient becomes pregnant while taking Scemblix. The pregnancy status of females of reproductive potential should be verified prior to starting treatment with Scemblix. Sexually-active females of reproductive potential should use effective contraception during treatment with Scemblix and for at least 3 days after the last dose (see Section 4.6 Fertility, Pregnancy and Lactation).
Use in hepatic impairment.
See Section 5.2 Pharmacokinetic Properties, Special populations.
Use in renal impairment.
See Section 5.2 Pharmacokinetic Properties, Special populations.
Use in the elderly.
See Section 5.2 Pharmacokinetic Properties, Special populations.
Paediatric use.
The safety and efficacy of Scemblix in paediatric patients (below 18 years) has not been established.
Effects on laboratory tests.
See Section 4.8 Adverse Effects (Undesirable Effects).4.5 Interactions with Other Medicines and Other Forms of Interactions
Agents that may increase asciminib plasma concentrations.
Strong CYP3A4 inhibitors.
Physiologically-based pharmacokinetic (PBPK) models predict that co-administration of Scemblix at 200 mg twice daily with a strong CYP3A4 inhibitor (clarithromycin) would increase asciminib AUCtau and Cmax by 77% and 49%, respectively.
Caution should be exercised during concomitant administration of Scemblix 200 mg twice daily with strong CYP3A4 inhibitors including but not limited to clarithromycin, telithromycin, troleandomycin, itraconazole, ketoconazole, voriconazole, ritonavir, indinavir, nelfinavir or saquinavir. Dose adjustment of Scemblix is not required.
Agents that may decrease asciminib plasma concentrations.
Strong CYP3A4 inducers.
Co-administration of a strong CYP3A4 inducer (rifampicin) decreased asciminib AUCinf by 14.9%, while increasing asciminib Cmax by 9% in healthy subjects receiving a single Scemblix dose of 40 mg. Co-administration of a strong CYP3A4 inducer (phenytoin) decreased asciminib AUCinf and Cmax by 34% and 22%, respectively, in healthy subjects receiving a single Scemblix dose of 200 mg.
Caution should be exercised during concomitant administration of Scemblix at all recommended doses with strong CYP3A4 inducers, including but not limited to carbamazepine, phenobarbital, phenytoin or St. John's wort (Hypericum perforatum). Dose adjustment of Scemblix is not required.
Agents that may have their plasma concentrations altered by asciminib.
CYP3A4 substrates with narrow therapeutic index.
Asciminib is a CYP3A4 inhibitor. Concomitant use of Scemblix increases the Cmax and AUC of CYP3A4 substrates, which may increase the risk of adverse reactions of these substrates.
Co-administration of asciminib with a CYP3A4 substrate (midazolam) increased midazolam AUCinf and Cmax by 28% and 11%, respectively, in healthy subjects receiving Scemblix 40 mg twice daily.
PBPK models predict that co-administration of asciminib at 80 mg once daily would increase midazolam AUCinf and Cmax by 24% and 17%, respectively, while co-administration of asciminib at 200 mg twice daily would increase midazolam AUCinf and Cmax by 88% and 58%, respectively.
Caution should be exercised during concomitant administration of Scemblix at all recommended doses with CYP3A4 substrates known to have a narrow therapeutic index, including, but not limited to, the CYP3A4 substrates fentanyl, alfentanil, dihydroergotamine, or ergotamine. Dose adjustment of Scemblix is not required.
CYP2C9 substrates.
Asciminib is a CYP2C9 inhibitor. Concomitant use of Scemblix increases the Cmax and AUC of CYP2C9 substrates, which may increase the risk of adverse reactions of these substrates.
Co-administration of asciminib with a CYP2C9 substrate (warfarin) increased S-warfarin AUCinf and Cmax by 41% and 8%, respectively, in healthy subjects receiving Scemblix 40 mg twice daily.
PBPK models predict that co-administration of asciminib at 80 mg once daily would increase S-warfarin AUCinf and Cmax by 52% and 4%, respectively, while co-administration of asciminib at 200 mg twice daily would increase S-warfarin AUCinf and Cmax by 314% and 7%, respectively.
Caution should be exercised during concomitant administration of Scemblix at 80 mg total daily dose with CYP2C9 substrates known to have a narrow therapeutic index, including, but not limited to phenytoin or warfarin. Dose adjustment of Scemblix is not required.
Concomitant administration of Scemblix at 200 mg twice daily with CYP2C9 sensitive substrates and CYP2C9 substrates known to have a narrow therapeutic index should be avoided and alternative medications should be considered. If co-administration cannot be avoided, the CYP2C9 substrates dose should be reduced. If co-administration with warfarin cannot be avoided, the frequency of international normalized ratio (INR) monitoring should be increased as the anti-coagulant effect of warfarin may be enhanced.
Substrates of OATP1B or BCRP.
Asciminib is an OATP1B and BCRP inhibitor. The effect of concomitant use of Scemblix with OATP1B and BCRP substrates has not been established in clinical studies. However, based upon a mechanistic understanding of the elimination of asciminib and its in vitro inhibitory potential, concomitant use of Scemblix increases the Cmax and AUC of OATP1B and BCRP substrates, which may increase the risk of adverse reactions of these substrates (see Section 5.1 Pharmacodynamic Properties).
Co-administration of asciminib at 80 mg once daily with an OATP1B, CYP3A4 and P-gp substrate (atorvastatin) increased atorvastatin AUCinf and Cmax by 14% and 24%, respectively, in healthy subjects.
Avoid concomitant administration of Scemblix at all recommended doses with rosuvastatin and consider alternative statins. If co-administration cannot be avoided, rosuvastatin dose should be reduced, as recommended in its product information.
Caution should be exercised during concomitant administration of Scemblix at all recommended doses with substrates of OATP1B, BCRP or both transporters, including, but not limited to sulfasalazine, methotrexate, pravastatin, atorvastatin, and simvastatin. Refer to OATP1B and BCRP substrates' dose reductions, as recommended in their prescribing information. Closely monitor for adverse reactions during concomitant use of Scemblix at all recommended doses.
P-gp substrates of narrow therapeutic index.
PBPK models predict that co-administration of asciminib at 40 mg twice daily and 80 mg once daily with a P-gp substrate (digoxin) would increase digoxin Cmax by 30% and 38% and AUCinf by 20% and 22%, respectively, while co-administration of asciminib at 200 mg twice daily would increase digoxin Cmax and AUCinf by 62% and 40%, respectively.
Caution should be exercised during concomitant administration of Scemblix at all recommended doses with P-gp substrates known to have a narrow therapeutic index, including but not limited to digoxin, dabigatran, and colchicine.
In vitro evaluation of drug interaction potential.
In vitro, asciminib reversibly inhibits CYP3A4/5, CYP2C9, CYP2C8, CYP2B6, CYP2C19, UGT1A1 and UGT2B7.
Transporters.
Asciminib is a substrate of BCRP and P-gp. In vitro, asciminib inhibits BCRP, P-gp, OATP1B1, OATP1B3, OCT1, OCT2, OAT1, OAT3, MATE1, MATE2. Based on PBPK models, asciminib increased the exposure of P-gp, OATP1B and BCRP substrates (see ''Substrates of OATP1B, of BCRP or of both transporters''). The clinical relevance of the interaction with OCT1 is currently unknown at Scemblix 200 mg twice daily dosing.
Multiple pathways.
Asciminib is metabolised by several pathways including the CYP3A4, UGT2B7 and UGT2B17 enzymes and biliary secreted by the transporter BCRP.
Medicinal products inhibiting or inducing multiple pathways may alter Scemblix exposure.
Asciminib inhibits several pathways including CYP3A4, CYP2C9, OATP1B, P-gp and BCRP. Scemblix may increase the exposure of medicinal products, which are substrates of these pathways (see subsection ''Substrates of OATP1B, of BCRP or of both transporters'').
QT prolonging agents.
Caution should be exercised during concomitant administration of Scemblix at 80 mg total daily dose and medicinal products with a known risk of torsades de pointes, including, but not limited to, bepridil, chloroquine, clarithromycin, halofantrine, haloperidol, methadone, moxifloxacin or pimozide (see Section 5.2 Pharmacokinetic Properties).
Concomitant administration of Scemblix at 200 mg twice daily dose and medicinal products with a known risk of torsades de pointes should be avoided (see Section 5.2 Pharmacokinetic Properties).
Drug-food interactions.
The bioavailability of asciminib decreases on consumption of food (see Section 4.2 Dose and Method of Administration; Section 5.2 Pharmacokinetic Properties).4.6 Fertility, Pregnancy and Lactation
Effects on fertility.
There are no data on the effect of Scemblix on human fertility.
In the rat fertility study, asciminib administered via the oral route at doses of 200 mg/kg/day did not affect reproductive function in male and female rats. A slight effect on male sperm motility and sperm count was observed at doses of 200 mg/kg/day, likely at AUC exposures 19-fold, 13-fold or 2-fold higher than those achieved in patients at the 40 mg twice-daily, 80 mg once-daily or 200 mg twice daily doses, respectively.
(Category D)
Risk summary.
Based on findings from animal studies, Scemblix can cause fetal harm when administered to a pregnant woman. There are no adequate and well-controlled studies in pregnant women to inform a product-associated risk.
Animal reproduction studies in pregnant rats and rabbits demonstrated that oral administration of asciminib during organogenesis induced embryotoxicity, fetotoxicity and teratogenicity. In embryofetal development studies, pregnant animals received oral doses of asciminib at 25, 150 and 600 mg/kg/day in rats and at 15, 50 and 300 mg/kg/day in rabbits during the period of organogenesis.
In rats, increases in fetal malformations (anasarca, cleft palate, cardiomegaly, pericardium filled with fluid and stenosis of the aortic arch) were noted at 150 mg/kg/day (AUC exposures 13-fold higher than those achieved in patients at the 40 mg twice daily or 80 mg once-daily doses) and increased fetal weights at 25 mg/kg/day (AUC exposures equal to those achieved in patients at the 40 mg twice daily or 80 mg once daily doses) in the absence of adverse maternal effects. At the fetal maternal no-observed-adverse-effect level of 25 mg/kg/day, the AUC exposures were below those achieved in patients at the 200 mg twice daily dose.
In rabbits, dosed at 50 mg/kg/day (AUC exposures 4-fold higher than those achieved in patients at the 40 mg twice daily or 80 mg once daily doses), increased incidence of resorptions, indicative of embryofetal mortality and incidence of cardiac malformations (including dilated aorta/aortic arch, truncus arteriosus, valve absent, ventricular septum defect and/or atretic pulmonary artery, indicative of dysmorphogenesis) were observed. At the fetal maternal no-observed-adverse-effect level of 15 mg/kg/day, the AUC exposures were below those achieved in patients at the 200 mg twice daily dose.
All these effects were in absence of adverse maternal effects and were considered to be drug related.
Pregnant women and females of reproductive potential should be advised of the potential risk to a fetus if Scemblix is used during pregnancy or if the patient becomes pregnant while taking Scemblix (see Section 4.4 Special Warnings and Precautions for Use).
Pregnancy testing.
The pregnancy status of females of reproductive potential should be verified prior to starting treatment with Scemblix.
Contraception.
Sexually-active females of reproductive potential should use effective contraception (methods that result in less than 1% pregnancy rates) during treatment with Scemblix and for at least 3 days after the last dose.
It is not known if asciminib is transferred into human milk after administration of Scemblix. There are no data on the effects of asciminib on the breastfed child or on milk production.
Because of the potential for serious adverse drug reactions in the breastfed child, breast-feeding is not recommended during treatment with Scemblix and for at least 3 days after the last dose.4.7 Effects on Ability to Drive and Use Machines
The effects of this medicine on a person's ability to drive and use machines were not assessed as part of its registration.
4.8 Adverse Effects (Undesirable Effects)
Summary of the safety profile.
The overall safety profile of Scemblix has been evaluated in 556 patients with Ph+ CML in chronic (CP) and accelerated (AP) phases receiving Scemblix as monotherapy. It is based on the safety pool of the pivotal phase III study J12301 (ASC4FIRST) (N=200 newly diagnosed Ph+CML-CP patients), the pivotal phase III study A2301 (ASCEMBL) (N=156 Ph+ CML-CP patients previously treated with 2 or more TKIs) and the phase I study X2101, including patients with: Ph+ CML-CP (N=115), Ph+ CML-CP harbouring the T315I mutation (N=70), Ph+ CML-AP (N=15).
The safety pool (N=556) includes patients receiving Scemblix at doses ranging from 10 to 200 mg twice daily and 80 to 200 mg once daily. In the pooled dataset, the median duration of exposure to Scemblix was 83.29 weeks (range: 0.1 to 439 weeks), with 79.3% of patients exposed for at least 48 weeks and 42.4% of patients exposed for at least 96 weeks, respectively.
The most common adverse drug reactions of any grade (incidence ≥ 20%) in patients receiving Scemblix were musculoskeletal pain (32.9%), thrombocytopenia (28.1%), fatigue (25%), upper respiratory tract infections (23.7%), headache (21.8%), neutropenia (21.6%), and diarrhoea (20%). The most common adverse drug reactions of ≥ grade 3 (incidence ≥ 5%) in patients receiving Scemblix were thrombocytopaenia (16.5%), neutropaenia (13.7%), increased pancreatic enzymes (9.4%) and hypertension (8.6%).
Serious adverse drug reactions occurred in 9.5% of patients receiving Scemblix. The most frequent serious adverse drug reactions (incidence ≥ 1%) were pleural effusion (1.6%), lower respiratory tract infections (1.4%), thrombocytopaenia (1.3%), pancreatitis (1.1%), pyrexia (1.1%).
Tabulated summary of adverse drug reactions from clinical trials.
Adverse drug reactions from clinical studies (Table 3) are listed by MedDRA system organ class. Within each system organ class, the adverse drug reactions are ranked by frequency, with the most frequent reactions first. Within each frequency grouping, adverse drug reactions are presented in order of decreasing seriousness. In addition, the corresponding frequency category for each adverse drug reaction is based on the following convention (CIOMS III): very common (≥ 1/10); common (≥ 1/100 to < 1/10); uncommon (≥ 1/1,000 to < 1/100); rare (≥ 1/10,000 to < 1/1,000); very rare (< 1/10,000).
 In the ASCEMBL study, decrease in phosphate levels occurred as a laboratory abnormality in 17.9% (all grades) and 7.1% (grade 3/4) of 156 patients receiving Scemblix at 40 mg twice daily. In the ASC4FIRST study, decrease in phosphate levels based on normal ranges occurred as a laboratory abnormality in 13% (all grades) of 200 patients receiving Scemblix at 80 mg once daily.
In the ASCEMBL study, decrease in phosphate levels occurred as a laboratory abnormality in 17.9% (all grades) and 7.1% (grade 3/4) of 156 patients receiving Scemblix at 40 mg twice daily. In the ASC4FIRST study, decrease in phosphate levels based on normal ranges occurred as a laboratory abnormality in 13% (all grades) of 200 patients receiving Scemblix at 80 mg once daily.
Description of selected adverse drug reactions.
Myelosuppression.
Thrombocytopaenia occurred in 156 of 556 (28.1%) patients receiving Scemblix, with grade 3 and 4 reactions reported in 39 (7%) and 53 (9.5%) of patients, respectively. Among the patients with thrombocytopaenia ≥ grade 3, the median time to first occurrence of reactions was 6 weeks (range: 0.14 to 64.14 weeks) with median duration of any occurring reaction of 1.57 weeks (95% CI, range: 1.14 to 2 weeks). Scemblix was permanently discontinued in 11 (2%) patients, while it was temporarily withheld in 70 (12.6%) patients due to thrombocytopenia.
Neutropaenia occurred in 120 of 556 (21.6%) patients receiving Scemblix, with grade 3 and 4 reactions reported in 41 (7.4%) and 35 (6.3%) patients, respectively. Among the patients with neutropaenia ≥ grade 3, the median time to first occurrence of reactions was 7.07 weeks (range: 0.14 to 180.14 weeks) with median duration of any occurring reaction of 1.86 weeks (95% CI, range: 1.29 to 2 weeks). Scemblix was permanently discontinued in 7 (1.3%) patients, while it was temporarily withheld in 52 (9.4%) patients due to neutropenia.
Anaemia occurred in 70 of 556 (12.9%) patients receiving Scemblix, with grade 3 reactions occurring in 22 (4%) patients. Among the patients with anaemia grade 3, the median time to first occurrence of reactions was 22.21 weeks (range: 0.14 to 207 weeks) with median duration of any occurring reaction of 0.79 weeks (95% CI, range: 0.4 to 2.1 weeks). Scemblix was temporarily withheld in 2 (0.4%) patients due to anaemia.
Reporting suspected adverse effects.
Reporting suspected adverse reactions after registration of the medicinal product is important. It allows continued monitoring of the benefit-risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions at www.tga.gov.au/reporting-problems.4.9 Overdose
There is limited experience of Scemblix overdose. In clinical studies, Scemblix has been administered at doses up to 280 mg twice daily with no evidence of increased toxicity. General supportive measures and symptomatic treatment should be initiated in cases of suspected overdose.
For information on the management of overdose, contact the Poisons Information Centre on 13 11 26 (Australia).
5 Pharmacological Properties
5.1 Pharmacodynamic Properties
Pharmacotherapeutic group: Antineoplastic agents, protein kinase inhibitors. ATC code: L01EA06.
Mechanism of action.
Asciminib is an oral and potent inhibitor of ABL/BCR::ABL1 tyrosine kinases. Asciminib inhibits the ABL1 kinase activity of the BCR::ABL1 fusion protein, by specifically targeting the ABL myristoyl pocket.
Pharmacodynamics (PD).
In vitro, asciminib inhibits the tyrosine kinase activity of ABL1 at mean IC50 values below 3 nanomolar. In patient-derived cancer cells, asciminib specifically inhibits the proliferation of cells harbouring BCR::ABL1 with IC50 values between 1 and 25 nanomolar. In cells engineered to express the wild-type or the T315I mutant form of BCR::ABL1, asciminib inhibits cell growth with mean IC50 value of 0.61 ± 0.21 and 7.64 ± 3.22 nanomolar, respectively.
In mouse xenograft models of CML, asciminib dose-dependently inhibited the growth of tumours harbouring either the wild-type or the T315I mutant form of BCR::ABL1, with tumour regression being observed at doses above 7.5 mg/kg or 30 mg/kg twice daily, respectively.
Cardiac electrophysiology.
Scemblix treatment is associated with an exposure-related prolongation of the QT interval. The correlation between asciminib concentration and the estimated maximum mean change from baseline of the QT interval with Fridericia's correction (ΔQTcF) was evaluated in 239 patients with Ph+ CML or Ph+ acute lymphoblastic leukaemia (ALL) receiving Scemblix at doses ranging from 10 to 280 mg twice daily and 80 to 200 mg once daily. The estimated mean ΔQTcF was 3.35 ms (upper bound of 90% CI: 4.43 ms) for Scemblix 40 mg twice-daily dose and 3.64 ms (upper bound of 90% CI: 4.68 ms), for the 80 mg once-daily dose and 5.37 ms (upper bound of 90% CI: 6.77 ms) for the 200 mg twice daily dose.
Clinical trials.
The clinical efficacy and safety of Scemblix in the treatment of patients with newly diagnosed Philadelphia chromosome-positive myeloid leukemia in chronic phase (Ph+ CML-CP) were demonstrated in the multi-center, randomized, active-controlled and open-label phase III study ASC4FIRST.
In this study, a total of 405 patients were randomized in a 1:1 ratio to receive either Scemblix or investigator selected tyrosine kinase inhibitors (IS-TKIs). Prior to randomization, the investigator selected the TKI (imatinib or second generation [2G] TKI) to be used in the event of randomization to the comparator arm, based on patient characteristics and comorbidities. Patients were stratified according to EUTOS long-term survival (ELTS) risk group (low, intermediate, high), and pre-randomization selection of TKI (imatinib or 2G TKIs stratum). Patients received either Scemblix or IS-TKIs, and continued treatment until unacceptable toxicity or treatment failure occurred.
Patients were 36.8% female and 63.2% male, with median age 51 years (range: 18 to 86 years). Of the 405 patients, 23.5% were 65 years or older, while 6.2% were 75 years or older. Patients were Caucasian (53.8%), Asian (44.4%), Black (1%) and 0.7% unknown. The demographic characteristics within the imatinib (N=203) and the 2G TKIs (N=202) strata were:
Median age: 55 years and 43 years, respectively;
ELTS high risk group: 8.4% and 13.9%, respectively;
Framingham cardiovascular disease high risk group: 35.5% and 17.8%, respectively.
The demographic characteristics were balanced across Scemblix and IS-TKIs, as well as across the two arms within the imatinib and 2G TKIs strata.
Of the 405 patients, 200 received Scemblix, while 201 received IS-TKIs. Of the 201 patients receiving IS-TKIs, 99 received imatinib, 49 received nilotinib, 42 received dasatinib, and 11 received bosutinib. Four patients did not receive any treatment.
The median duration of treatment was 69.8 weeks (range: 0.7 to 107.7 weeks) for patients receiving Scemblix and 64.3 weeks (range: 1.3 to 103.1 weeks) for patients receiving IS-TKIs. By 48 weeks, 90% of patients on Scemblix and 80.6% of patients on IS-TKIs were still receiving treatment.
The study had multiple primary objectives assessing major molecular response rate (MMR) at 48 weeks. One primary objective evaluated Scemblix compared to IS-TKIs. The other primary objective evaluated Scemblix compared to IS-TKIs, within the imatinib stratum. A secondary objective evaluated MMR at 48 weeks evaluating Scemblix compared to IS-TKIs, within the 2G TKIs stratum.
The main efficacy outcomes from ASC4FIRST are summarized in Table 4.
 The predicted MMR rate at 48 weeks for the Scemblix 40 mg twice-daily dose is comparable to the MMR rate at 48 weeks observed in ASC4FIRST with the Scemblix 80 mg once-daily dose, based on exposure-response analysis.
The predicted MMR rate at 48 weeks for the Scemblix 40 mg twice-daily dose is comparable to the MMR rate at 48 weeks observed in ASC4FIRST with the Scemblix 80 mg once-daily dose, based on exposure-response analysis.
Median time to MMR in patients receiving Scemblix, IS-TKIs, IS-TKIs within the imatinib stratum, and IS-TKIs within the 2G TKIs stratum were: 24.3 weeks (95% CI: 24.1 to 24.6 weeks), 36.4 weeks (95% CI: 36.1 to 48.6 weeks), 48.6 weeks (95% CI: 36.1 to 59.6 weeks), and 36.1 weeks (95% CI: 24.4 to 48.1 weeks), respectively.
MMR rates at 48 weeks by ELTS risk group in patients receiving Scemblix, IS-TKIs, IS-TKIs within the imatinib stratum, and IS-TKIs within the 2G TKIs stratum were: 72.1%, 57.6%, 50% and 65.6% for low risk, respectively; 64.3%, 36.8%, 26.7% and 48.2% for intermediate risk, respectively; 52.2%, 31.8%, 12.5% and 42.9% for high risk, respectively.
By 48 weeks, MR4.0 achieved by patients receiving Scemblix, IS-TKIs, IS-TKIs within the imatinib stratum, and IS-TKIs within the 2G TKIs stratum was: 40.8%, 22.1%, 15.7%, and 28.4%, respectively. By 48 weeks, MR4.5 achieved by patients receiving Scemblix, IS-TKIs, IS-TKIs within the imatinib stratum and IS-TKIs within 2G TKIs stratum was: 19.9%, 11.8%, 5.9%, and 17.7%, respectively.
Ph+ CML-CP, previously treated with two or more TKIs.
The clinical efficacy of Scemblix in the treatment of patients with Ph+ CML-CP previously treated with two or more tyrosine kinase inhibitors were demonstrated in the multi-centre, randomised, active-controlled and open-label phase III study ASCEMBL.
In this study, a total of 233 patients were randomised in a 2:1 ratio and stratified according to major cytogenetic response (MCyR) status at baseline to receive either Scemblix 40 mg twice daily (N=157) or bosutinib 500 mg once daily (N=76). Patients continued treatment until unacceptable toxicity or treatment failure occurred.
Patients with Ph+ CML-CP previously treated with two or more TKIs were 51.5% female and 48.5% male with median age 52 years (range: 19 to 83 years). Of the 233 patients, 18.9% were 65 years or older, while 2.6% were 75 years or older. Patients were Caucasian (74.7%), Asian (14.2%) and Black (4.3%). Of the 233 patients, 80.7% and 18% had Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1, respectively. Patients who had previously received 2, 3, 4, 5 or more prior lines of TKIs were 48.1%, 31.3%, 14.6% and 6%, respectively. The median duration of treatment was 156 weeks (range: 0.1 to 256.3 weeks) for patients receiving Scemblix and 30.5 weeks (range: 1 to 293.3 weeks) for patients receiving bosutinib.
The primary endpoint of the study was MMR at 24 weeks and the key secondary endpoint was MMR rate at 96 weeks. MMR is defined as BCR::ABL1 ratio ≤ 0.1% by International Scale [IS]. Secondary endpoints were complete cytogenetic response rate (CCyR) at 24 and 96 weeks, defined as no Philadelphia-positive metaphases in bone marrow with a minimum of 20 metaphases examined.
The main efficacy outcomes from ASCEMBL are summarised in Table 5.
 The Kaplan-Meier estimated OS rate at 2 years was 97.3% (95% CI: 92.9, 99.0) for the asciminib arm and 98.6% for the bosutinib arm (95% CI: 90.2, 99.8).
The Kaplan-Meier estimated OS rate at 2 years was 97.3% (95% CI: 92.9, 99.0) for the asciminib arm and 98.6% for the bosutinib arm (95% CI: 90.2, 99.8).
The MMR rate at 24 weeks in patients in whom the randomised treatment represented the third, fourth, fifth or more line of TKI was 29.3%, 25%, and 16.1% in patients treated with Scemblix and 20%, 13.8%, and 0% in patients receiving bosutinib, respectively.
The MMR rate at 48 weeks was 29.3% (95% CI: 22.32, 37.08) in patients receiving Scemblix and 13.2% (95% CI: 6.49, 22.87) in patients receiving bosutinib. The Kaplan-Meier estimated proportion of patients receiving Scemblix and maintaining MMR for at least 120 weeks was 97% (95% CI: 88.6, 99.2).
Ph+ CML-CP harbouring the T315I mutation.
The clinical efficacy of Scemblix in the treatment of patients with Ph+ CML-CP with the T315I mutation was evaluated in a multi-centre open-label study CABL001X2101 (NCT02081378). Testing for T315I mutation utilised a qualitative p210 BCR::ABL1 mutation test using Sanger Sequencing.
Efficacy was based on 48 patients with Ph+ CML-CP with the T315I mutation who received Scemblix at a dose of 200 mg twice daily. Patients continued treatment until unacceptable toxicity or treatment failure occurred.
Of the 48 patients, 77.1% were male and 22.9% female; 33.3% were 65 years or older, while 8.3% were 75 years or older with a median age of 56.5 years (range, 26 to 86 years). The patients were White (47.9%), Asian (25%), and Black or African American (2.1%), and 25% were unreported or unknown. Seventy-five percent and 25% of patients had ECOG performance status 0 and 1, respectively. Patients who had previously received 1, 2, 3, 4, and 5 or more TKIs were 16.7%, 31.3%, 35.4%, 14.6%, and 2%, respectively.
MMR was achieved by 24 weeks in 42% (19/45, 95% CI: 27.7-57.8) of the 45 patients treated with Scemblix. MMR was achieved by 96 weeks in 49% (22/45, 95% CI: 34% to 64%) of the 45 patients treated with Scemblix. The median duration of treatment was 108 weeks (range, 2 to 215 weeks).
5.2 Pharmacokinetic Properties
Absorption.
Asciminib is rapidly absorbed, with median maximum plasma levels (Tmax) reached 2 to 3 hours after oral administration, independent of the dose. The geometric mean (geoCV%) of Cmax at steady state is 1781 nanogram/mL (23%) and 793 nanogram/mL (49%) following administration of Scemblix at 80 mg once-daily and 40 mg twice-daily doses, respectively. The geometric mean (geoCV%) of Cmax at steady state is 5642 nanogram/mL (40%) following administration of Scemblix at 200 mg twice daily dose. The geometric mean (geoCV%) of AUCtau is 5262 nanogram*h/mL (48%) following administration of Scemblix at 40 mg twice-daily dose.
PBPK models predict that the asciminib absorption is approximately 100%, while bioavailability is approximately 73%.
Asciminib bioavailability may be reduced by co-administration of oral medicinal products containing hydroxypropyl-β-cyclodextrin as an excipient. Co-administration of multiple doses of itraconazole oral solution containing hydroxypropyl-β-cyclodextrin at a total of 8 g per dose with a 40 mg dose of asciminib, decreased asciminib AUCinf by 40.2% in healthy subjects.
Food effect.
Food consumption decreases asciminib bioavailability, with a high-fat meal having a higher impact on asciminib pharmacokinetics than a low-fat meal. Asciminib AUC is decreased by 62.3% with a high-fat meal and by 30% with a low-fat meal compared to the fasted state, independent of the dose (see Section 4.2 Dose and Method of Administration; Section 4.5 Interactions with Other Medicines and Other Forms of Interactions).
Distribution.
Asciminib apparent volume of distribution at steady state is 111 L, based on population pharmacokinetic analysis. Asciminib is mainly distributed to plasma, with a mean blood-to-plasma ratio of 0.58, independent of the dose. Asciminib is 97.3% bound to human plasma proteins, independent of the dose.
Metabolism.
Asciminib is primarily metabolised via CYP3A4-mediated oxidation (36%), UGT2B7- and UGT2B17-mediated glucuronidation (13.3% and 7.8%, respectively). Asciminib is the main circulating component in plasma (92.7% of the administered dose).
Excretion.
Asciminib is mainly eliminated via fecal excretion, with a minor contribution of the renal route. Eighty and 11% of the asciminib dose were recovered in the feces and in the urine of healthy subjects, respectively, following oral administration of a single 80 mg dose of [14C]-labelled asciminib. Fecal elimination of unchanged asciminib accounts for 56.7% of the administered dose.
The oral total clearance (CL/F) of asciminib is 6.31 L/hour, based on population pharmacokinetic analysis. The accumulation half-life of asciminib is 5.2 hours at 40 mg twice daily and 80 mg once daily.
PBPK models predict that asciminib biliary secretion via BCRP accounts for 31.1% of its total systemic clearance.
Linearity/non-linearity.
Asciminib exhibits a slight dose over-proportional increase in steady-state exposure (AUC and Cmax) across the dose range of 10 to 200 mg administered once or twice daily.
The geometric mean average accumulation ratio is approximately 2-fold, independent of the dose. Steady-state conditions are achieved within 3 days at the 40 mg twice-daily dose.
Special populations.
Use in elderly patients (65 years of age or above).
Among the 556 patients receiving Scemblix in the ASC4FIRST, ASCEMBL and X2101 studies, 130 (23.4%) patients were 65 years or older, and 31 (5.6%) were 75 years or older.
No overall differences in the safety or efficacy of Scemblix were observed between patients of 65 years of age or above and younger patients. There is an insufficient number of patients of 75 years of age or above to assess whether there are differences in safety or efficacy.
Gender/race/body weight.
Asciminib systemic exposure is not affected by gender, race or body weight to any clinically relevant extent.
Renal impairment.
A dedicated renal impairment study including 6 subjects with normal renal function (absolute glomerular filtration rate [aGFR] ≥ 90 mL/min) and 8 subjects with severe renal impairment not requiring dialysis (aGFR 15 to < 30 mL/min) has been conducted. Asciminib AUCinf and Cmax are increased by 56% and 8%, respectively, in subjects with severe renal impairment compared to subjects with normal renal function, following oral administration of a single 40 mg dose of Scemblix (see Section 4.2 Dose and Method of Administration).
Population pharmacokinetics models indicate an increase in asciminib median steady state AUC0-24h by 11.5% in subjects with mild to moderate renal impairment, compared to subjects with normal renal function.
Hepatic impairment.
A dedicated hepatic impairment study including 8 subjects each with normal hepatic function, mild hepatic impairment (Child-Pugh A score 5 to 6), moderate hepatic impairment (Child-Pugh B score 7 to 9) or severe hepatic impairment (Child-Pugh C score 10 to 15) was conducted. Asciminib AUCinf is increased by 22%, 3% and 66% in subjects with mild, moderate and severe hepatic impairment, respectively, compared to subjects with normal hepatic function, following oral administration of a single 40 mg dose of Scemblix (see Section 4.2 Dose and Method of Administration).
5.3 Preclinical Safety Data
Asciminib was evaluated in safety pharmacology, repeated dose toxicity, genotoxicity, carcinogenicity, reproductive toxicity and phototoxicity studies.
Genotoxicity.
Asciminib was negative for genotoxicity in a bacterial reverse mutation assay, in vitro micronucleus assays in lymphocytes and TK6 cells and in vivo, in a rat micronucleus assay at PO doses up to 600 mg/kg/day.
Carcinogenicity.
The carcinogenic potential of asciminib was investigated in a 2-year study by the oral route in rats. Benign ovarian Sertoli cell tumours were observed in female animals at 66 mg/kg/day (yielding exposure to asciminib 8- and 6-fold higher than in patients at 40 mg twice daily and 80 mg once daily, respectively, and comparable to that in patients with dosing at 200 mg twice daily, based on plasma AUC). This occurred in conjunction with increased ovarian Sertoli cell hyperplasia (observed with treatment at ≥ 30 mg/kg/day). The clinical relevance of the finding is currently unknown. No treatment-related neoplastic or hyperplastic findings were observed in male rats up to the highest dose tested (200 mg/kg/day; yielding exposure 18, 12 and 2.5 times higher, respectively, than in patients treated at 40 mg twice daily, 80 mg once daily and 200 mg twice daily).6 Pharmaceutical Particulars
6.1 List of Excipients
Scemblix tablets contain the following inactive ingredients:
20 mg film-coated tablets.
Lactose monohydrate, microcrystalline cellulose (E460i), hydroxypropylcellulose (E463), croscarmellose sodium (E468), polyvinyl alcohol (E1203), titanium dioxide (E171), magnesium stearate, talc (E553b), colloidal silicon dioxide, iron oxide (E172, yellow and red), lecithin (E322), xanthan gum (E415).
40 mg film-coated tablets.
Lactose monohydrate, microcrystalline cellulose (E460i), hydroxypropylcellulose (E463), croscarmellose sodium (E468), polyvinyl alcohol (E1203), titanium dioxide (E171), magnesium stearate, talc (E553b), colloidal silicon dioxide, iron oxide (E172, black and red), lecithin (E322), xanthan gum (E415).
6.2 Incompatibilities
Incompatibilities were either not assessed or not identified as part of the registration of this medicine.
6.3 Shelf Life
In Australia, information on the shelf life can be found on the public summary of the Australian Register of Therapeutic Goods (ARTG). The expiry date can be found on the packaging.
6.4 Special Precautions for Storage
Store below 25°C - blister packs.
Store below 30°C - bottles.
Store in the original package in order to protect from moisture.
6.5 Nature and Contents of Container
Scemblix tablets are supplied in HDPE bottles or PCTFE/PVC/Alu blisters.*
Scemblix 20 mg tablets.
Supplied in blister packs or bottles containing 20 or 60 tablets.*
Scemblix 40 mg tablets.
Supplied in blister packs or bottles containing 20 or 60 tablets or in bottles containing 300 tablets.*
*Not all pack sizes and container types may be marketed.
6.6 Special Precautions for Disposal
In Australia, any unused medicine or waste material should be disposed of by taking to your local pharmacy.
6.7 Physicochemical Properties
Asciminib hydrochloride has a molecular formula C20H18ClF2N5O3.HCl; the free base has a molecular weight of 449.8. Asciminib HCl is a crystalline powder with pKa 3.9 and pH-dependent solubility.
Chemical structure.

CAS number.
2119669-71-3.7 Medicine Schedule (Poisons Standard)
Schedule 4 - Prescription Only Medicine.
Summary Table of Changes



 The recommended dosage modification for the management of selected adverse drug reactions is shown in Table 2.
The recommended dosage modification for the management of selected adverse drug reactions is shown in Table 2.
 In the ASCEMBL study, decrease in phosphate levels occurred as a laboratory abnormality in 17.9% (all grades) and 7.1% (grade 3/4) of 156 patients receiving Scemblix at 40 mg twice daily. In the ASC4FIRST study, decrease in phosphate levels based on normal ranges occurred as a laboratory abnormality in 13% (all grades) of 200 patients receiving Scemblix at 80 mg once daily.
In the ASCEMBL study, decrease in phosphate levels occurred as a laboratory abnormality in 17.9% (all grades) and 7.1% (grade 3/4) of 156 patients receiving Scemblix at 40 mg twice daily. In the ASC4FIRST study, decrease in phosphate levels based on normal ranges occurred as a laboratory abnormality in 13% (all grades) of 200 patients receiving Scemblix at 80 mg once daily. The predicted MMR rate at 48 weeks for the Scemblix 40 mg twice-daily dose is comparable to the MMR rate at 48 weeks observed in ASC4FIRST with the Scemblix 80 mg once-daily dose, based on exposure-response analysis.
The predicted MMR rate at 48 weeks for the Scemblix 40 mg twice-daily dose is comparable to the MMR rate at 48 weeks observed in ASC4FIRST with the Scemblix 80 mg once-daily dose, based on exposure-response analysis. The Kaplan-Meier estimated OS rate at 2 years was 97.3% (95% CI: 92.9, 99.0) for the asciminib arm and 98.6% for the bosutinib arm (95% CI: 90.2, 99.8).
The Kaplan-Meier estimated OS rate at 2 years was 97.3% (95% CI: 92.9, 99.0) for the asciminib arm and 98.6% for the bosutinib arm (95% CI: 90.2, 99.8).
